Article contents
Molten salt synthesis of color-tunable and single-component NaY(1−x−y)(WO4)2:Eu3+ x ,Tb3+ y phosphor for UV LEDs
Published online by Cambridge University Press: 16 February 2017
Abstract
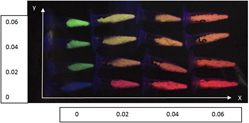
Tungstate based phosphors have efficient absorption in the UV region and can be used for UV-pumped light emitting. For novel and effective materials and synthesis methods in this system, a series of Eu3+ and Tb3+ co-doped NaY(WO4)2 phosphors have been synthesized via the molten salt method. The powder X-ray diffraction (PXRD) patterns, scanning electronic microscope (SEM), and photoluminescent spectra have been characterized for the prepared samples. The results show the flux (NaCl) not only decreases the reaction temperature (700–900 °C) than the normal solid state synthesis (∼1000 °C), but also controls the morphology of the products. The shape and size of products can be changed simply and effectively by the reaction conditions, such as temperature and heating time. It is also found that the emission colors of the samples can be tuned from red to green by simply adjusting the doping concentrations of Eu3+ and Tb3+ ions under the same wave length excitation, which has potential applications for multi-color display and illumination as a single-component phosphor.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
These authors contributed equally to this work.
Contributing Editor: Winston V. Schoenfeld
References
REFERENCES
- 4
- Cited by





