Article contents
Methanol desorption in poly(methyl methacrylate) with stress distributions
Published online by Cambridge University Press: 26 September 2014
Abstract
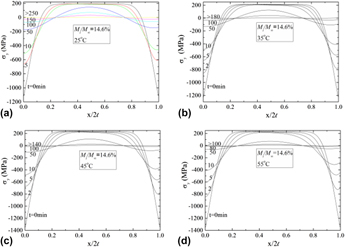
Understanding solvent transport in polymers is of practical importance for the applications of polymers in the fields of food packaging, biomedical bandages, materials engineering, etc. We studied one-side desorption in poly(methyl methacrylate) (PMMA). Experimental results showed that methanol desorption in PMMA depended on temperature and the initial distribution of concentration. The diffusion coefficient in PMMA and the evaporation rate of methanol across the PMMA surface followed the Arrhenius relation. The activation energies for the diffusion and the evaporation of methanol are 18.3, 42.6 and 8.6, 18.3 kJ/mol for the specimens with the ratio of initial mass to the equilibrium saturated absorbed mass, Mi/M∞, being 14.6% and 35.3%, respectively. The partial molal volume increased with the increase of the desorption temperature for Mi/M∞ = 14.6%, while it had an opposite trend for Mi/M∞ = 35.5%. The chemical stresses developed in PMMA during the desorption were also studied.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 4
- Cited by




