Article contents
In situ single-step reduction of bromine-intercalated graphite to covalently brominated and alkylated/brominated graphene
Published online by Cambridge University Press: 20 May 2020
Abstract
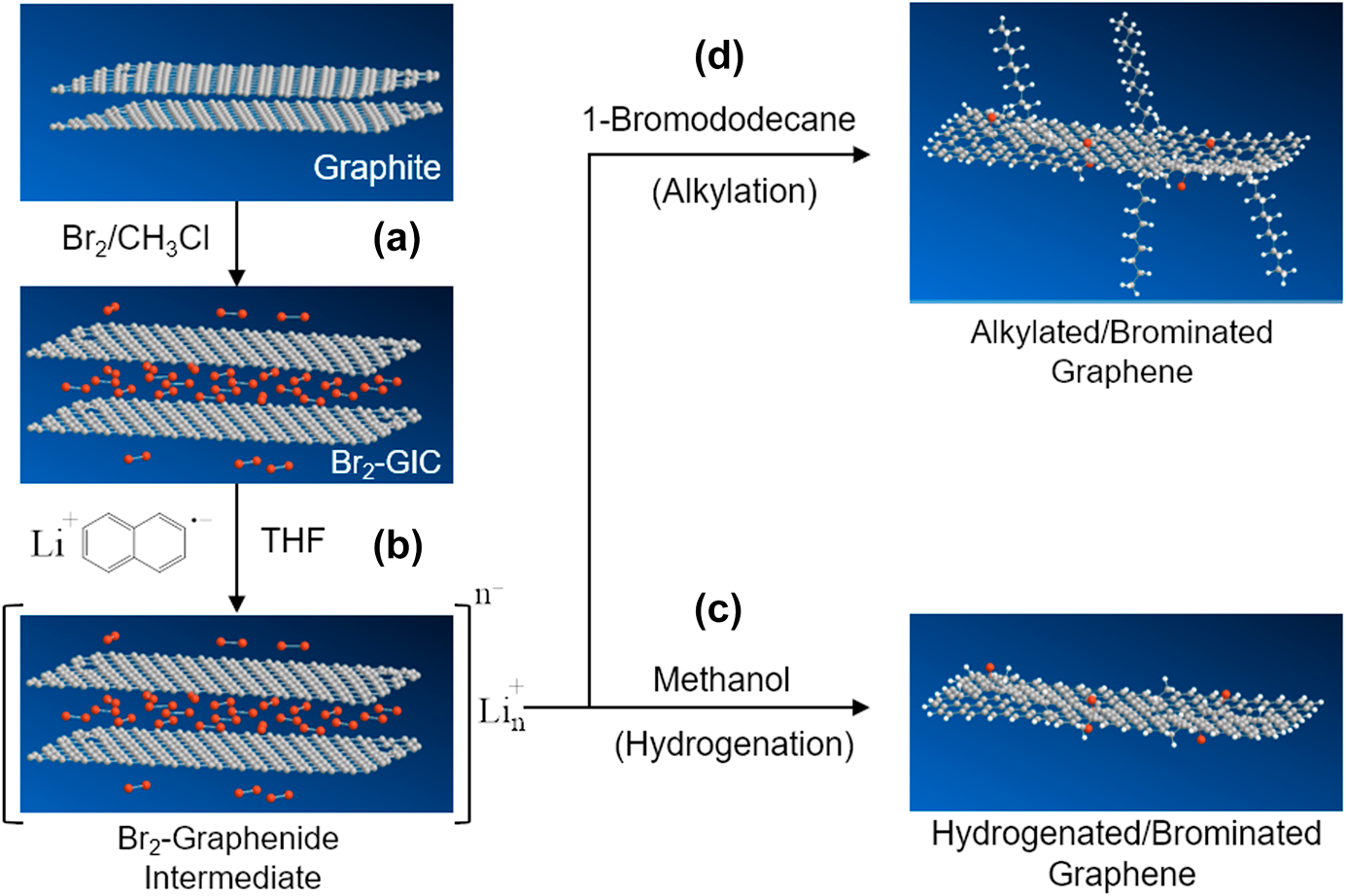
Developing easy and effective surface functionalization approaches has required to facilitate the processability of graphene while seeking novel application areas. Herein, an in situ single-step reductive covalent bromination of graphene has been reported for the first time. Highly brominated graphene flakes (>3% Br) were prepared by only subjecting the bromine-intercalated graphite flakes to a reduction reaction with reactive lithium naphthalide. The bromine-functionalized graphene was characterized by X-ray photoelectron spectroscopy and thermogravimetric analysis. Results revealed that Br2 molecules acted as both an intercalating agent for the graphite and a reactant for the surface functionalization of the graphene. After brominating, the remaining negative charges on the reduced graphene surface were further used for the dual surface functionalization of graphene with a long-chain alkyl group (~1% dodecyl group addition). The functionalized graphenes were also characterized by Fourier transform infrared and Raman spectroscopy.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 11: Focus Section: Heterogeneity in Beyond Graphene 2D Materials , 15 June 2020 , pp. 1472 - 1480
- Copyright
- Copyright © Materials Research Society 2020
References
- 5
- Cited by


