Article contents
Impact of linker positions for thieno[3,2-b]thiophene in wide band gap benzo[1,2-b:4,5-b′]dithiophene-based photovoltaic polymers
Published online by Cambridge University Press: 12 March 2019
Abstract
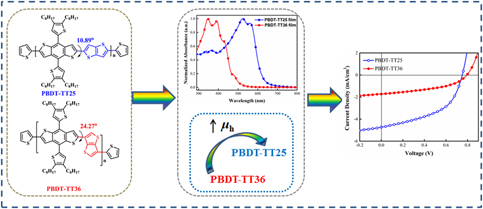
Two wide band gap conjugated polymers, namely PBDT-TT25 and PBDT-TT36, derived from (4,8-bis(4,5-dioctyl-thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl)bis(trimethylstannane) with 2,5-dibromothieno[3,2-b]thiophene (TT25) or 3,6-dibromothieno[3,2-b]thiophene (TT36), have been synthesized by simply altering the linker positions of thieno[3,2-b]thiophene unit. The impact of linker positions on the energy levels, aggregation, active layer morphology, and optical and photovoltaic properties was evaluated systemically. We found that the absorption was greatly broadened, and the highest occupied molecular orbital (HOMO) energy level was elevated as the result of the significantly reduced twist angle on the polymer backbone when the linker positions changed from 3,6-isomer to 2,5-isomer. Therefore, the optimal inverted polymer solar cells exhibited a 1.87 times enhancement in power conversion efficiencies (PCE), which was mainly ascribed to the higher short circuit current densities (JSC) and fill factor (FF) of the devices mainly benefited from the widened, stronger absorption, higher hole mobility, and more ordered structure.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 2
- Cited by


