Published online by Cambridge University Press: 21 January 2019
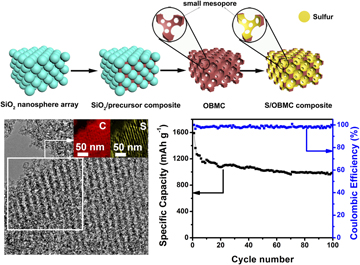
3D ordered bimodal mesoporous carbon (OBMC) with a high specific surface area of 1368.7 m2/g, ordered large mesopores, and small mesopores on the walls is prepared by a surfactant-free rapid method using SiO2 nanosphere arrays as templates. The resulting OBMC is then composited with sulfur to prepare S/OBMC hybrids via a simple solution infiltration method followed by a heat treatment process. In S/OBMC composite, sulfur is uniformly infiltrated inside the 3D hierarchical pores of OBMC. On the basis of this systematic design, the obtained S/OBMC cathode shows a large discharge capacity value of 1590 mA h/g at first cycle and maintains 989 mA h/g after 100 cycles at 0.2 C. Furthermore, at 1 C charge–discharge rate, a reversible discharge capacity of 733 mA h/g after 100 cycles is reached. The extraordinary electrochemical property of S/OBMC derives from the unique bimodal mesoporous structure with large mesopores and small mesopores that can facilitate the mass transfer and strict dissolution of polysulfide species into the electrolyte.