Article contents
Hierarchical ZnO films with microplate/nanohole structures induced by precursor concentration and colloidal templates, their superhydrophobicity, and enhanced photocatalytic performance
Published online by Cambridge University Press: 03 July 2013
Abstract
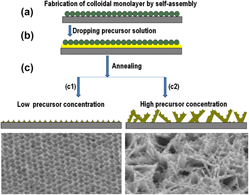
A facile inexpensive route has been developed to prepare ZnO hierarchical materials with microplate/nanohole structures based on the colloidal monolayer template by the precursor thermal decomposition. These hierarchical structured materials demonstrated an excellent superhydrophobicity with self-cleaning effect and an enhanced photocatalytic performance to organic molecules, which are attributed to big roughness and large surface area of such special hierarchical structures. The formation mechanism of such hierarchical structures was investigated in detail by tracing morphology changing at different precursor concentrations. At high precursor concentration, both incompletely restricted ZnO growth of colloidal templates and preferable growth of microplates take place at the same time, and hence, ZnO hierarchical materials with microplate/nanohole structures are formed. With increasing precursor concentration, the number density of ZnO microplates tends to be larger. The large number density of ZnO microplates and holes on the microplates render the sample a large surface area and surface roughness, leading to good superhydrophobicity and photocatalytic activity. Such hierarchical ZnO micro/nanostructured materials have important applications in environmental science, microfluidic devices, etc.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 29 , Issue 1: Focus Issue: Synthesis of Nanostructured Functional Oxides , 14 January 2014 , pp. 115 - 122
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 10
- Cited by




