Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Yuan, Lu
Yin, Qiyue
Wang, Yiqian
and
Zhou, Guangwen
2013.
CuO reduction induced formation of CuO/Cu2O hybrid oxides.
Chemical Physics Letters,
Vol. 590,
Issue. ,
p.
92.
Yuan, Lu
Cai, Rongsheng
Jang, Joon I.
Zhu, Wenhui
Wang, Chao
Wang, Yiqian
and
Zhou, Guangwen
2013.
Morphological transformation of hematite nanostructures during oxidation of iron.
Nanoscale,
Vol. 5,
Issue. 16,
p.
7581.
Yuan, Lu
Wang, Chao
Cai, Rongsheng
Wang, Yiqian
and
Zhou, Guangwen
2013.
Spontaneous ZnO nanowire formation during oxidation of Cu-Zn alloy.
Journal of Applied Physics,
Vol. 114,
Issue. 2,
Zhao, C. X.
Li, Y. F.
Zhou, J.
Li, L. Y.
Deng, S. Z.
Xu, N. S.
and
Chen, Jun
2013.
Large-Scale Synthesis of Bicrystalline ZnO Nanowire Arrays by Thermal Oxidation of Zinc Film: Growth Mechanism and High-Performance Field Emission.
Crystal Growth & Design,
Vol. 13,
Issue. 7,
p.
2897.
Zhu, Jian
Ng, K. Y. Simon
and
Deng, Da
2014.
Micro Single Crystals of Hematite with Nearly 100% Exposed {104} Facets: Preferred Etching and Lithium Storage.
Crystal Growth & Design,
Vol. 14,
Issue. 6,
p.
2811.
Lin, Dong
Deng, Biwei
Sassman, Stephen A.
Hu, Yaowu
Suslov, Sergey
and
Cheng, Gary J.
2014.
Magnetic field assisted growth of highly dense α-Fe2O3 single crystal nanosheets and their application in water treatment.
RSC Adv.,
Vol. 4,
Issue. 36,
p.
18621.
Yuan, Lu
Wang, Chao
Cai, Rongsheng
Wang, Yiqian
and
Zhou, Guangwen
2014.
Temperature-dependent growth mechanism and microstructure of ZnO nanostructures grown from the thermal oxidation of zinc.
Journal of Crystal Growth,
Vol. 390,
Issue. ,
p.
101.
Zhu, Huihui
Deng, Jinxia
Chen, Jun
Yu, Ranbo
and
Xing, Xianran
2014.
Growth of hematite nanowire arrays during dense pentlandite oxidation.
Journal of Materials Chemistry A,
Vol. 2,
Issue. 9,
p.
3008.
Deng, Da
2015.
Li‐ion batteries: basics, progress, and challenges.
Energy Science & Engineering,
Vol. 3,
Issue. 5,
p.
385.
Kotenev, V. A.
Kiselev, M. R.
Vysotskii, V. V.
Averin, A. A.
and
Tsivadze, A. Yu.
2016.
The formation of urchinlike nanostructures under thermal oxidation and depassivation of iron particles.
Protection of Metals and Physical Chemistry of Surfaces,
Vol. 52,
Issue. 5,
p.
825.
Zhu, Wenhui
Winterstein, Jonathan P
Sharma, Renu
and
Zhou, Guangwen
2016.
The Growth of Catalyst-free NiO Nanowires.
Microscopy and Microanalysis,
Vol. 22,
Issue. S3,
p.
1620.
Zhu, Wenhui
Winterstein, Jonathan
Maimon, Itai
Yin, Qiyue
Yuan, Lu
Kolmogorov, Aleksey N.
Sharma, Renu
and
Zhou, Guangwen
2016.
Atomic Structural Evolution during the Reduction of α-Fe2O3 Nanowires.
The Journal of Physical Chemistry C,
Vol. 120,
Issue. 27,
p.
14854.
Feng, Honglei
Wang, Yiqian
Wang, Chao
Diao, Feiyu
Zhu, Wenhui
Mu, Peng
Yuan, Lu
Zhou, Guangwen
and
Rosei, Federico
2016.
Defect-induced enhanced photocatalytic activities of reducedα-Fe2O3nanoblades.
Nanotechnology,
Vol. 27,
Issue. 29,
p.
295703.
Zhu, Wenhui
Winterstein, Jonathan P.
Yang, Wei-Chang David
Yuan, Lu
Sharma, Renu
and
Zhou, Guangwen
2017.
In Situ Atomic-Scale Probing of the Reduction Dynamics of Two-Dimensional Fe2O3 Nanostructures.
ACS Nano,
Vol. 11,
Issue. 1,
p.
656.
Kotenev, V. A.
2018.
Regularities of Vacuum Oxidation of Iron in the Range of Low-Temperature Passivation According to the Data of Spectral Ellipsometry.
Protection of Metals and Physical Chemistry of Surfaces,
Vol. 54,
Issue. 5,
p.
969.
Zhu, Jian
and
Deng, Da
2018.
Anisotropic Particle Assemblies.
p.
261.
Sun, Xianhu
Zhu, Wenhui
Wu, Dongxiang
Liu, Zhenyu
Chen, Xiaobo
Yuan, Lu
Wang, Guofeng
Sharma, Renu
and
Zhou, Guangwen
2020.
Atomic‐Scale Mechanism of Unidirectional Oxide Growth.
Advanced Functional Materials,
Vol. 30,
Issue. 4,
Zhu, Dingding
Wang, Xinli
Zhao, Jun
Lu, Jian
Zhou, Yichun
Cai, Canying
Huang, Jianyu
and
Zhou, Guangwen
2020.
Effect of water vapor on high-temperature oxidation of NiAl alloy.
Corrosion Science,
Vol. 177,
Issue. ,
p.
108963.
Lai, Ming-Wei
and
Kurata, Hiroki
2021.
Exploring (1$$\overline{1}$$2)-related ordered structure in oxidation-synthesized α-Fe2O3 nanowhiskers.
Journal of Materials Science,
Vol. 56,
Issue. 12,
p.
7286.
Lee, Seonyong
and
Jang, Ho Won
2021.
α-Fe2O3 nanostructure-based gas sensors.
JOURNAL OF SENSOR SCIENCE AND TECHNOLOGY,
Vol. 30,
Issue. 4,
p.
210.
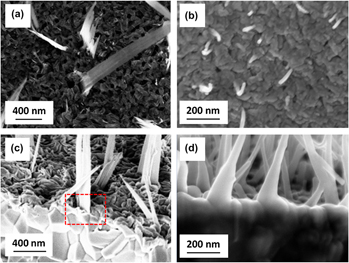
 direction with a bicrystal structure. It is shown that nanowires are rooted on Fe2O3 grains, whereas nanobelts are originated from the boundaries of Fe2O3 grains. Our results show that oxygen gas pressure can be used to manipulate the Fe2O3/Fe3O4 interfacial reaction, thereby tailoring the oxide growth morphologies via the stress-driven diffusion.
direction with a bicrystal structure. It is shown that nanowires are rooted on Fe2O3 grains, whereas nanobelts are originated from the boundaries of Fe2O3 grains. Our results show that oxygen gas pressure can be used to manipulate the Fe2O3/Fe3O4 interfacial reaction, thereby tailoring the oxide growth morphologies via the stress-driven diffusion.

