Article contents
General strategy for doping rare earth metals into Au–ZnO core–shell nanospheres
Published online by Cambridge University Press: 02 December 2019
Abstract
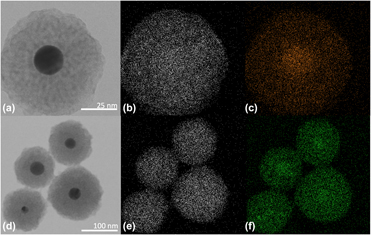
Multifunctional nanoparticles are an emerging area of research, impacting numerous fields ranging from biomedical applications to energy. While initial core–shell structures consisted of similar materials, such as Au–Ag or CdTe–CdSe nanoparticles, recent work has expanded this line of investigation to include particles of dissimilar materials. However, there are several challenges when synthesizing dissimilar material systems. In this work, a method for doping the shell of an Au–ZnO nanosphere is demonstrated. Several metal dopants are investigated, including Cu, Ce, Er, Nd, Tm, and Yb. The ZnO shell is nucleated on the gold nanosphere core via an ascorbic acid–assisted growth, and the dopant is intercalated uniformly into the shell during the self-assembly phase of the shell formation. The doping and polycrystalline shell are confirmed using a series of qualitative and quantitative methods. This multi-material nanoparticle synthesis strategy opens the door for future applications in sensing, photocatalysis, and bioimaging.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 3
- Cited by




