Article contents
Effect of Ti6Al4V surface morphology on the osteogenic differentiation of human embryonic stem cells
Published online by Cambridge University Press: 26 October 2017
Abstract
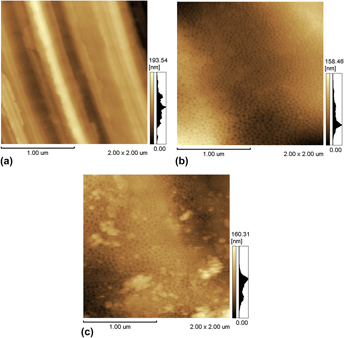
Ti6Al4V alloys usually need to undergo some kind of surface treatment to enable good bone growth and implant integration. In this work, three treatments that modify the titanium alloy surface were studied with the aim of promoting osteogenic differentiation of human-embryonic-stem-cell-derived mesenchymal progenitors (hESC-MPs). The surface treatments used were mechanical polishing and electropolishing for 4 or 12 min in an H2SO4/HF/glycerine solution. The samples were characterised by atomic force microscopy, profilometry, X-ray photoelectron spectroscopy, and wettability. Samples were seeded with hESC-MPs in osteogenic media, and the cell number and alkaline phosphatase activity were measured. The electropolishing surface treatments influenced the nanometric morphology and wettability. However, the electropolished surfaces contributed in the same way as the mechanically polished surface to osteogenic differentiation, indicating that differentiation was strongly influenced by microroughness, which did not differ among the treatments used in the present work.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Lakshmi Nair
References
REFERENCES
- 6
- Cited by


