Article contents
Effect of charge density on water sorption and elasticity of stimuli-responsive poly(acrylamide–itaconic acid) and poly(N,N-dimethylacrylamide–itaconic acid) hydrogels: Comparison of experiment with theory
Published online by Cambridge University Press: 25 November 2013
Abstract
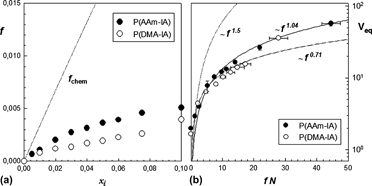
In this study, stimuli-responsive ionic poly(acrylamide–itaconic acid) (P(AAm–IA)) and poly(N,N-dimethylacrylamide–itaconic acid) (P(DMA–IA)) hydrogels have been prepared by free radical crosslinking copolymerization in aqueous solution using N,N-methylenebisacrylamide as the crosslinking agent. In particular, the swelling ratio and elasticity of both hydrogel systems including the effect of ionic comonomer itaconic acid (IA) content were investigated. In spite of the similarity in monomer/crosslinker ratio and the content of ionic comonomer in the hydrogel structures, comparable differences were observed in their swelling capacity and elasticity. Compared to P(DMA–IA) hydrogels, P(AAm–IA) hydrogels exhibit higher swelling capacity in water and a more pronounced dependency of the swelling ratio on the ionic comonomer content. The incorporation of a small amount of IA into the network structure causes the hydrogel system to exhibit polyelectrolyte type swelling behavior. P(AAm–IA) and P(DMA–IA) hydrogels showed good response to the valency of counterions and pH of the external solution.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 8
- Cited by




