Article contents
Corrosion of nickel and nickel–phosphorous-coated AISI 430 in dry (Ar–3%H2) and humid hydrogen (Ar–3%H2–3%H2O) atmosphere
Published online by Cambridge University Press: 01 September 2020
Abstract
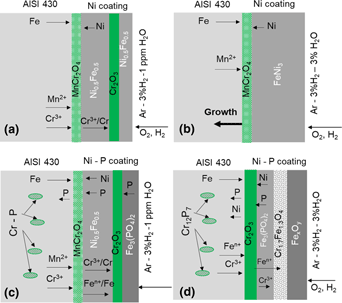
The corrosion behavior of uncoated, nickel (Ni) and nickel–phosphorous (Ni–P)-coated AISI 430 alloy was investigated in Ar–3%H2 and Ar–3%H2–3%H2O atmosphere at 800 °C for 100 h. Microstructure, chemical composition, and reaction products were analyzed by scanning electron microscopy, energy-dispersive spectroscopy, and X-ray diffraction techniques. The corrosion extent of Ni–P-coated AISI 430 is higher than Ni-coated AISI 430. Oxidation promotes corrosion in the uncoated and coated alloy. The oxidation rate of Ni-coated alloy is the lowest in Ar–3%H2 but Ni–P-coated alloy in Ar–3%H2–3%H2O for initial 20 h. The oxidation rate of the Ni–P-coated sample is ~14 times higher in 20–100 h in Ar–3%H2–3%H2O. External growth of Cr2O3 is observed for Ni-coated alloy in Ar–3%H2 and for Ni–P-coated alloy in both the atmospheres. Inward growth of Cr2O3 by AISI 430 alloy consumption attributes to the lowest oxidation rate and the corrosion extent of Ni-coated sample in Ar–3%H2–3%H2O.
- Type
- Invited Feature Paper
- Information
- Copyright
- Copyright © Materials Research Society 2020
Footnotes
These authors contributed equally to this work.
References
- 3
- Cited by





