Article contents
Controlled synthesis of ytterbium ion and erbium ion codoped gadolinium oxyfluoride hollow nanosphere with upconversion luminescence property
Published online by Cambridge University Press: 06 March 2013
Abstract
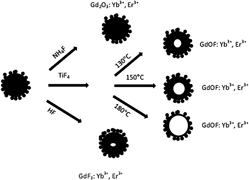
Uniform ytterbium ion and erbium ion codoped gadolinium oxyfluoride (GdOF: Yb3+, Er3+) hollow nanospheres of 100-nm diameter were synthesized via the nanoscale Kirkendall approach, using colloidal nanospheres of ytterbium ion and erbium ion codoped gadolinium hydroxide [Gd(OH)3: Yb3+, Er3+] as sacrificial templates and titanium tetrafluoride as fluorine source under hydrothermal condition. The shell thickness of the as-synthesized GdOF: Yb3+, Er3+ hollow nanospheres can be facilely tuned from 31 to 13 nm by controlling reaction temperature and reaction time. The upconversion emission color could be adjusted from red to yellow to green when the host lattices variedfrom gadolinium (III) oxide to gadolinium oxyfluoride to gadolinium fluoride. Furthermore, the formation mechanism of the hollow GdOF: Yb3+, Er3+ nanospheres was found to depend on the fluorine source.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 6
- Cited by


