Article contents
Synthesis of LiV3O8 nanorods and shape-dependent electrochemical performance
Published online by Cambridge University Press: 01 January 2011
Abstract
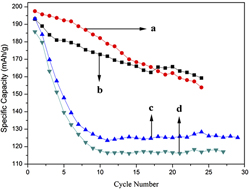
Shape control of nanocrystals has become an indispensable part in material research, such as developing new battery raw materials and synthesizing high activity catalysts. In this work, one-dimensional LiV3O8 nanorods have been fabricated by high temperature solid-state reaction using V2O5 nanowires as precursors obtained via a hydrothermal method. The as-prepared LiV3O8 nanorods were characterized by x-ray diffraction, transmission electron microscopy, scanning electron microscopy, and galvanostatic tests, compared with LiV3O8 samples synthesized by the traditional one-step solid-state method. The results show that LiV3O8 nanorods exhibited better electrochemical performance than those synthesized by the traditional method, indicating that a different shape will lead to huge distinctions in electrochemical properties. This work demonstrates that Li-insertion/deintercalation dynamics might be crystal morphology-sensitive.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 3
- Cited by




