Article contents
Novel growth mode of solid–liquid–solid (SLS) silica nanowires
Published online by Cambridge University Press: 23 June 2011
Abstract
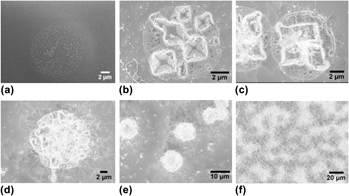
A novel and previously unreported, high temperature solid–liquid–solid (SLS) silica nanowire (NW) growth mode has been observed and investigated. In this mode, SLS NW nucleation and subsequent growth was uniquely promoted by—and coupled to—the formation of thermally etched pyramidal pits in the Si substrate that formed during a high temperature anneal phase before the onset of SLS NW formation. The silicon oxide-mediated thermal pit formation process enhanced Si transport to Au–Si alloy droplets directly adjacent to the pyramidal pits. Consequently, SLS NW nucleation and growth was preferentially promoted at the pit edges. The promotion of SLS NW growth by the pyramidal pits resulted in the observation of SLS NW “blooms” at the pit locations. Subsequent NW growth, occurring both at the pit sites and from Au–Si alloy droplets distributed across the planar surfaces of the Si wafer, eventually occluded the pits. This newly observed process is termed as “thermal pit-assisted growth.”
- Type
- Articles
- Information
- Journal of Materials Research , Volume 26 , Issue 17: Focus Issue: Nanowires: Fundamentals and Applications , 14 September 2011 , pp. 2232 - 2239
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 8
- Cited by


