Article contents
A novel approach of preparing zinc adipate as β-nucleating agent for polypropylene engineering
Published online by Cambridge University Press: 20 September 2019
Abstract
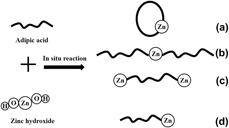
In this work, two types of zinc adipate β-nucleating agents, Adi-Zn(OH)2 (1:1) and Adi-ZnO (1:1), for polypropylene (PP) were prepared and their performances were evaluated and compared with commercial β-nucleating agent (named CNA). Results showed that Adi-Zn(OH)2 (1:1) was more effective in promoting PP to form β-crystals and improving the impact strength of PP in the range of nucleating agent addition (0–0.4 wt%). Based on these findings, the ratio of adipic acid to zinc hydroxide and the nonisothermal crystallization kinetics of the optimum ratio of adipic acid to zinc hydroxide were systematically studied; results showed that at 0.2 wt%, Adi-Zn(OH)2 (1:2), the nucleated PP displayed the highest impact strength, which was 2.6 times that of pure PP and 42% higher than that of CNA. Besides, Adi-Zn(OH)2 (1:2) could also afford to induce the formation of a high content of β-crystals and shorten the crystallization half time (t1/2) and accelerate the crystallization of PP.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 8
- Cited by


