Article contents
Nanocomposite polymer electrolyte membranes based on poly(vinylphosphonic acid)/TiO2 nanoparticles
Published online by Cambridge University Press: 05 December 2012
Abstract
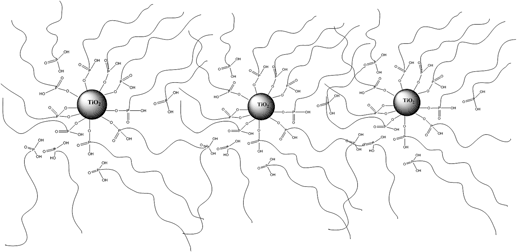
In the present work, proton conducting nanocomposite electrolytes containing poly(vinylphosphonic acid) (PVPA) and TiO2 have been prepared and characterized. In the nanocomposite materials, the TiO2 content was varied from 5% to 20% (w/w) to improve mechanical strength and stability. The thermal stability, proton conductivity, as well as morphology of the electrolytes were investigated. The interaction existed between PVPA and TiO2 was confirmed by Fourier transform infrared spectroscopy. Thermogravimetric analysis showed that the samples are thermally stable up to approximately 200 °C. Differential scanning calorimetry results indicate that the Tg of the materials shift to higher temperatures as TiO2 content increases. Scanning electron microscopy results confirmed that the TiO2 nanoparticles were successfully incorporated and homogeneously distributed in the PVPA matrix. In the anhydrous state, the proton conductivity of PVPA(10)TiO2(in situ) has been found to be 0.004 S/cm at 120 °C. The proton conductivity of these membranes are also measured and compared with previous studies.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 8
- Cited by


