Article contents
Development of the ReaxFF reactive force field for aluminum–molybdenum alloy
Published online by Cambridge University Press: 09 May 2013
Abstract
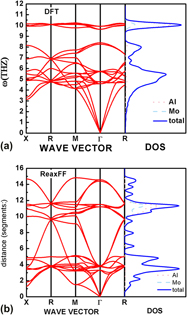
We have developed a reactive force field within the ReaxFF framework to accurately describe reactions involving aluminum–molybdenum alloy, which are part parameters of Al–O–Mo ternary system metastable intermolecular composites. The parameters are optimized from a training set, whose data come from density functional theory (DFT) calculations and experimental value, such as heat of formation, geometry data, and equation of states, which are reproduced well by ReaxFF. Body-centered cubic molybdenum’s surface energy, vacancy formation, and two transformational paths, Bain and trigonal paths are calculated to validate the ReaxFF ability describing the defects and deformations. Some structures’ elastic constant and phonon are calculated by DFT and ReaxFF to predict the structures’ mechanics and kinetic stability. All those results indicate that the fitted parameters can describe the energy difference of various structures under various circumstances and generally represent the diffusion property but cannot reproduce the elasticity and phonon spectra so well.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 10
- Cited by




