Article contents
Ce3+/Tb3+ activated GdF3, KGdF4, and CeF3 submicro/nanocrystals: Synthesis, phase evolution, and optical properties
Published online by Cambridge University Press: 23 November 2011
Abstract
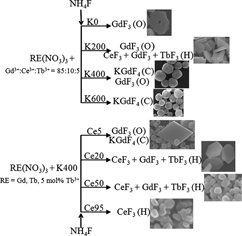
Based on a citric acid-assisted hydrothermal method, series of Ce3+/Tb3+ activated fluorides have been synthesized. By controlling the amount of KNO3, the final products evolve from the Ce3+/Tb3+ codoped orthorhombic phase GdF3 to the Ce3+/Tb3+ codoped cubic phase KGdF4. The concentration of Ce3+ has great effects on the crystalline phases and the morphologies of final products. The Ce3+ concentration dependent samples illustrate the appearance of the hexagonal phase solid solution CeF3–GdF3–TbF3 in the final products. When the Ce3+ concentration is 20 mol%, the sample Ce20 presents the hexagonal phase CeF3 but the diffraction peaks move to higher degree. The x-ray diffraction patterns suggest the phase evolution of final products, the field emission scanning electron microscopy images present the variation in morphology of samples, and the photoluminescence excitation and emission spectra as well as the luminescent dynamic curves illustrate the optical properties of samples.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 6
- Cited by




