Introduction
Ice cores from Greenland and Antarctica have provided crucial evidence with which to reconstruct the environmental and climatic past in polar regions (e.g. Reference Alley, Gow, Johnsen, Kipfstuhl, Meese and ThorsteinssonAlley and others, 1995; Reference McManusMcManus, 2004). To complement information obtained in these regions, it is essential to study ice cores in low- and mid-latitude glaciers. Although these cores cover shorter timescales, they contribute unique information on local and regional conditions (e.g. Reference Thompson, Mosley-Thompson and HendersonThompson and others, 2000, Reference Thompson, Mosley-Thompson, Davis, Lin, Henderson and Mashiotta2003).
Low- and mid-latitude glaciers are generally temperate, and traditional methods used in polar ice cannot normally be used for characterizing the stratigraphy and dating ice cores from these glaciers since annual and seasonal signals of stable isotopes and chemical species are strongly disturbed due to melt and percolation (Reference Schwikowski, Brütsch, Gäggeler and SchottererSchwikowski and others, 1999b; Reference Eichler, Schwikowski and GäggelerEichler and others, 2001; Reference Pohjola, Casassa, Sepúlveda and SinclairPohjola, 2002; Reference OlivierOlivier and others, 2003). Other methods of characterizing and dating ice cores from temperate glaciers are therefore needed.
The study of microorganisms living on temperate glaciers can provide a novel, robust method for ice-core analysis. Dating of temperate ice cores has been performed previously by analysis of snow algae, such as on Yala glacier, Nepal Himalaya (Reference Yoshimura, Kohshima, Takeuchi, Seko and FujitaYoshimura and others, 2000); Glaciar Tyndall, Hielo Patagónico Sur (southern Patagonia icefield) (Reference ShiraiwaShiraiwa and others, 2002; Reference KohshimaKohshima and others, 2007); and Sofiyskiy glacier, Russian Altai, where other biological components such as pollen and fungi have also been studied (Reference UetakeUetake and others, 2006). These investigations together with other studies of algal communities at the glacier surface concluded that snow algae are a useful seasonal marker for ice-core dating, reconstruction of past climate and environmental conditions, and assessment of past annual mass balance at low/mid-latitudes and low altitudes (e.g. Reference Yoshimura, Kohshima and OhtaniYoshimura and others, 1997; Reference Takeuchi, Kohshima and FujitaTakeuchi and others, 1998; Reference TakeuchiTakeuchi, 2001; Reference Kohshima, Yoshimura, Takeuchi, Casassa, Sepúlveda and SinclairKohshima and others, 2002; Reference Takeuchi and KohshimaTakeuchi and Kohshima, 2004).
Snow algae grow in the snow and ice during the melt season. They are well preserved in ice cores, as their size is larger than that of chemical species and isotopes, and are therefore less affected by percolation (Reference Yoshimura, Kohshima, Takeuchi, Seko and FujitaYoshimura and others, 2000; Reference Kohshima, Yoshimura, Takeuchi, Casassa, Sepúlveda and SinclairKohshima and others 2002; Reference ShiraiwaShiraiwa and others, 2002; Reference UetakeUetake and others, 2006). Because of their size (<30 μm), they are called microalgae hereafter.
Besides microalgae, various organisms have been found on the surface of several glaciers, such as insects, copepods, fungi, cyanobacteria and bacteria (Reference KohshimaKohshima, 1984; Reference TakeuchiTakeuchi, 2001; Reference Segawa, Miyamoto, Ushida, Agata, Okada and KohshimaSegawa and others, 2005; Reference UetakeUetake and others, 2006). This ecosystem is relatively simple and closed, comparable to other freshwater environments such as lakes and rivers where microalgae support heterotrophic populations (Reference Kohshima, Kawano, Connell and HidakaKohshima, 1987; Reference Kohshima, Yoshimura, Takeuchi, Casassa, Sepúlveda and SinclairKohshima and others, 2002).
New and alternative methods for dating temperate glaciers have focused on pollen seasonally dispersed on glaciers. For example, seasonal and annual markers have been detected based on pollen signals from ice cores from the Altai (Reference NakazawaNakazawa and others, 2004, Reference Nakazawa2005). Pollen can also be a sensitive indicator of past atmospheric circulation, being a valuable proxy for understanding the climatic and environmental past in polar and non-polar regions (Reference Liu, Yao and ThompsonLiu and others, 1998; Reference Bourgeois, Koerner, Gajewski and FisherBourgeois and others, 2000; Reference NakazawaNakazawa and others, 2004, Reference Nakazawa2005).
To improve the reliability of ice-core dating, multiple information sources are clearly beneficial, such as pollen and microalgae (Reference UetakeUetake and others, 2006). Furthermore, microorganisms growing on the glacier can also provide information on past environmental conditions, such as temperature and snow accumulation, which affect their growth (Reference Yoshimura, Kohshima, Takeuchi, Seko and FujitaYoshimura and others, 2000; Reference Kohshima, Yoshimura, Takeuchi, Casassa, Sepúlveda and SinclairKohshima and others, 2002).
The objective of this study is to date, by means of biological analyses, three shallow firn cores (∼10 m) retrieved from temperate glaciers in the Andes of the Chilean lake district. In addition to biological analyses, the preservation of chemical and isotopic records and physical properties is evaluated in order to date the ice cores with seasonal and annual resolution, and attempt to estimate the mass balance at each location. This study is also expected to aid in the selection of an appropriate site for obtaining a future deep ice core in the region (>50 m) which could provide a unique climatic and environmental record from mid-latitudes in the Southern Hemisphere.
Materials and Methods
Firn/ice core drilling and site description
Three firn cores were retrieved from glaciers located on volcanoes in the lake district of the Cordillera de los Andes, Chile (Fig. 1). At Volcán Mocho-Choshuenco (39°55′ S, 72°02′ W), one 10.0 m long core was drilled at 2000 m a.s.l. on the southeast glacier on 26 October 2005, and one 10.2 m long firn core was drilled the following day at 2422 ma.s.l. on the summit. On 1 November 2005, a 10.4 m long core was drilled on the summit of Volcán Osorno (41°06′ S,′ 72°30′ W; 2652 m a.s.l.). The thickness and position of ice lenses were recorded and firn/ice density was measured immediately after drilling.

Fig. 1. Location of Volcán Mocho-Choshuenco and Volcán Osorno in the Chilean lake district.
Both volcanoes are active, the last eruptions having occurred in 1864 at Volcán Mocho-Choshuenco and in 1869 at Volcán Osorno (Reference González-FerránGonzález-Ferrán, 1995). Both volcano summits are ice-filled calderas with relatively flat surfaces. The glaciological characteristics of Volcán Mocho-Choshuenco are well documented since 2003 as a result of a regular mass-balance monitoring programme (Reference Rivera, Bown, Casassa, Acuña and ClaveroRivera and others, 2005). The volcano summit altitudes were thought to be high enough to preserve some of the chemical and biological signals deposited on the glaciers. In fact, the mean annual temperature of coastal stations at latitudes similar to that of the volcanoes is ∼11°C. Assuming a vertical lapse rate of 6°C km−1, the expected mean annual temperature at the summit of the volcanoes should be −3 to −5°C. However, the presence of melt layers and thick ice lenses on the surface and in shallow pits detected during the glacier mass-balance measurements indicated that meltwater-related post-depositional processes might be relevant. Additionally, release of latent heat during refreeze periods can raise the firn/ice layer temperature to the melting point, and in fact these glaciers might be of temperate nature. Enhanced geothermal heat in this active volcanic region might also contribute to raising the ice temperature through thermal conductivity at the glacier bed, although there are no data to sustain this.
Volcán Mocho-Choshuenco and Volcán Osorno are stratovolcanoes approximately 20 km in diameter, composed of layers of lavas and pyroclasts and a summit caldera with a nested pyroclastic cone in the centre (Echegaray, 2000). These volcanic rocks were generated through a long sequence of various kinds of eruptions with ages ranging from early Pleistocene to late Holocene (AD 1864), corresponding mainly to lavas and in a minor proportion to pyroclasts, with an andesitic-basaltic to dacitic composition (Echegaray, 2000). The regional geological composition beyond the volcanic cones shows the presence of a variety of formations, including sedimentary, metamorphic, volcanic and intrusive rocks, with ages ranging from Upper Carboniferous to Quaternary.
Sample processing
On the glacier the firn/ice core samples were cut with a pre-cleaned stainless-steel knife into 20 cm sections, scraping off a 1 cm section of the core surface to eliminate possible contaminants. The samples were packed into pre-cleaned plastic bags and later melted at room temperature in a laboratory at Centro de Estudios Cientsíficos (CECS), Valdivia, Chile, each sample being bottled in two clean plastic containers of 50 mL each. All samples were then immediately stored in a freezer at −20°C. One bottle was used for biological analysis, and the other was sent to Japan for analyses of stable isotopes and chemical species.
Sample analysis
In the biological analyses performed at CECS, the samples were mounted and fixed inside a laminar-flow table. Filtering of 15 mL samples was performed using hydrophilic polytetrafluoroethylene (PTFE) membrane filters (JHWP013000:0.2 μm pore size, 13 mm diameter; Millipore, USA). Each filter was mounted and fixed in glycerol, formalin and water solution (1: 1: 1 volume) on a glass slide under cover-slip.
Counts of microorganisms and pollen grains were made with a fluorescent microscope (Olympus BX-FLA). The total cell number of microalgae and pollen of Nothofagus spp. was estimated on each filter by counting the cells along three to five parallel transects. In the case of Podocarpaceae, all pollen grains were counted within the filter. For protozoa the cell number was estimated along nine parallel transects across the filter.
The algae cells were classified according to their morpho-types, which correspond to microalgae showing the same morphological and size characteristics. Each morpho-type was assigned a capital letter. Mean cell volume was estimated by measuring the size of 50–100 cells for each morphotype, classifying their sizes in intervals of 1 μm. Algal biovolume was determined using the technique developed by Reference LohmannLohmann (1908), Reference Yoshimura, Kohshima and OhtaniYoshimura and others (1997, Reference Yoshimura, Kohshima, Takeuchi, Seko and Fujita2000) and Reference Hillebrand, Dürselen, Kirschstel, Pollingher and ZoharyHillebrand and others (1999). Microalgae smaller than 5 μm were eliminated in the algal biovolume results shown in the profiles, as suggested by Reference UetakeUetake and others (2006).
Pollen density was recorded according to the technique described in Reference NakazawaNakazawa and others (2004, Reference Nakazawa2005). The work of Reference HeusserHeusser (1971) and expert advice (personal communication from P. Moreno, 2006) were used for pollen identification.
Two control runs with five replicas each were performed to check for possible sources of contamination in the method. One control run used nanopure water frozen in bottles during a period of 1 week, and the other control run used nanopure water frozen during 1 month. The control runs were mounted, fixed and counted using the same method as for the samples.
Major ions of Na+, Ca2+, K+, NH4 +, NO3 −, Mg2+, SO4 2− and Cl− and stable isotopes of SD were analyzed at the Institute of Low Temperature Science, Hokkaido University, Japan, using ion chromatography and mass spectrometry (Reference Schwikowski, Döscher, Gäggeler and SchottererSchwikowski and others, 1999a, b; Reference ShiraiwaShiraiwa and others, 2002). The analysis of chemical species was not performed for the core drilled at 2000 m a.s.l. on Volcán Mocho-Choshuenco.
Results and Discussion
Organisms and pollen observed in the ice-core samples
Microalgae, protozoa, copepods and pollen grains in ice-core samples are described for the three cores.
Microalgae
Twenty-seven morphotypes of microalgae were observed in the ice core from Volcán Osorno. Twenty-five belong to Division Chlorophyta, and two to Division Cyanophyta. Maximum biovolume in the ice core is 19.9 × 104 μm3 mL−1 at 6 m depth, K and J being the largest biovolume morpho-types in this layer (Fig. 2).

Fig. 2. Largest biovolume morphotypes (K and J) of microalgae observed in the Volcán Osorno summit core. The photos on the left were acquired under a normal light microscope; those on the right under an Olympus BX-FLA fluorescence microscope.
Twenty-two morphotypes of microalgae were observed in the ice core from the summit of Volcán Mocho-Choshuenco. Twenty-one are from Division Chlorophyta, and one from Division Cyanophyta. Maximum biovolume in the ice core is 5.6 × 104 μm3 mL−1 at 7 m depth, N, K and FA being the largest biovolume morphotypes at this depth (Fig. 3).

Fig. 3. Largest biovolume morphotypes (FA, N and K) of microalgae observed in the Volcán Mocho-Choshuenco summit core. The photos on the left were taken under a light microscope; those on the right under an Olympus BX-FLA fluorescence microscope.
Seventeen morphotypes of microalgae were observed in the ice core from 2000 m a.s.l. at Volcán Mocho-Choshuenco. Sixteen are from Division Chlorophyta, and one from Division Cyanophyta. Maximum biovolume in the ice core is 1.4 × 103 μm3 mL−1.
Algal biovolume values from the volcano summits are similar to those reported in other studies of ice cores such as at Yala glacier (Reference Yoshimura, Kohshima, Takeuchi, Seko and FujitaYoshimura and others, 2000), Glaciar Tyndall (Reference ShiraiwaShiraiwa and others, 2002; Reference KohshimaKohshima and others, 2007) and Sofiyskiy glacier (Reference UetakeUetake and others, 2006).
Protozoa
Undescribed species of protozoa (Reference SantibáñezSantibáñez, 2007) were found in the cores from Osorno and Mocho-Choshuenco volcanoes. These were used as an additional tool for biological interpretation. These protozoa were confirmed to live on the glacier, as was inferred from observation of individuals present in the samples which showed undigested food inside the testa and also signals of reproduction.
Maximum protozoa abundance was 3 individuals mL−1 in the Volcán Osorno core, 38 individuals mL−1 in the Mocho-Choshuenco summit core and 1.6 individuals mL−1 in the Mocho-Choshuenco 2000 m core.
Copepods
Small copepods of about 800 μm in body length, and a nauplius (larval stage) of about 100 μm in body length, were observed in the bottom ice layer of the Volcán Mocho-Choshuenco 2000 m core. This finding suggests that copepods live and reproduce on the glacier. The morphology of this copepod is close to that of the copepod species living in the snow and ice of a Himalayan glacier as reported by Reference KikuchiKikuchi (1994).
Pollen
Podocarpaceae and genus Nothofagus spp. (Fig. 4) are the most abundant in all cores. The presence of these pollen types is in agreement with the predominant trees growing in the surrounding Valdivian forest and the anemophilian characteristics shown by both types of pollen. Flowering timing of genus Nothofagus depends on each species, with periods between August and December (Reference Riveros, Smith-Ramirez, Armesto, Villagrán and ArroyoRiveros and Smith-Ramirez, 1995; Reference Riveros, Smith-Ramirez, Armesto, Villagrán and ArroyoRiveros and others, 1995; Reference Riveros, Pérez, Báez and BáezRiveros and others, 2003; Reference HechenleitnerHechenleitner and others, 2005). Podocarpaceae family members flower between November and January (Reference HechenleitnerHechenleitner and others, 2005). The flowering period of tree species occurs later at higher altitudes, continuing until late summer in some cases (personal communication from P. Moreno, 2006).

Fig. 4. Pollen observed in the Volcán Osorno and Volcán Mocho-Choshuenco cores: (a, b) Podocarpaceae pollen; and (c, d) Nothofagus spp. pollen. Photos (a) and (c) were taken under a light microscope, (d) under an Olympus BX-FLA fluorescence microscope, and (b) under a scanning electron microscope.
Pollen peaks of Podocarpaceae and Nothofagus are absent in some layers estimated to correspond to spring–summer from biological profiles at Osorno and MochoChoshuenco volcanoes. Previous data show that pollen deposits on the surface of tropical glaciers from the Andean altiplano in Bolivia and Peru present significant spatial and temporal variations, showing differences in pollen concentration and percentage at the same sites, and between two consecutive years (Reference Reese, Liu and MountainReese and others, 2003; Reference Reese and LiuReese and Liu, 2005). If relevant temporal and/or spatial variations occur in pollen, due to either source or post-depositional processes, this dating method becomes unreliable (Reference NakazawaNakazawa and others, 2005). Therefore, knowledge of the spatial and/or temporal variability of pollen on the glacier surface is needed before it is validated as a reliable seasonal or annual record. Consequently, the absence of pollen peaks in some summer–spring layers interpreted from microalgae and protozoa does not necessarily connote periods without pollen dispersal.
Maximum Podocarpaceae pollen concentration in the Volcán Osorno core is 1.47 grains mL−1, and maximum Nothofagus spp. pollen concentration is 0.74 grains mL−1. In the Volcán Mocho-Choshuenco summit core, maximum Podocarpaceae pollen concentration is 0.93 grains mL−1 and maximum Nothofagus spp. pollen concentration is 0.41 grains mL−1. For the Mocho-Choshuenco 2000 m core, maximum Podocarpaceae pollen concentration is 1.8 grains mL−1 and maximum Nothofagus spp. pollen concentration is 0.2 grains mL−1.
Volcán Osorno core analysis
Variations in the biological profile, physical properties, δD and ion chemistry
The vertical profiles of microorganisms show preserved fluctuations at Volcán Osorno (Fig. 5). The vertical algal biovolume shows two peaks at 5.6–7.2 m and 8.2–10.0 m that are interpreted as corresponding to the meltwater season. These levels are in agreement with those with maximum protozoa abundance.

Fig. 5. Profiles of the Volcán Osorno core: (a) algal biovolume; (b) protozoa abundance; (c) Podocarpacea pollen concentration; (d) Nothofagus spp. pollen concentration; (e) ice-layer percentage; (f) firn density; (g) Cl−/Na+ ratio (vertical line corresponds to the marine reference (Reference Keene, Pszenny, Galloway and HawleyKeene and others, 1986)); (h) deuterium; (i) chloride; (j) sodium; (k) calcium; (l) magnesium; (m) potassium; (n) sulphate; (o) nitrate; and (p) ammonium. The lower grey shaded areas indicate the algal growth periods (summer: S) estimated from microalgal peaks. The upper grey shaded area indicates the pollen flowering period (spring: Sp) estimated from pollen peaks. The autumn–winter periods (in white) are labelled A–W.
A Podocarpaceae-rich layer appears within the surface layer from 0 to 1 m, and a Nothofagus-rich layer appears from 0 to 0.5 m in depth (Fig. 5c and d). This surface layer is interpreted as corresponding to the pollen dispersal season, which agrees with the core-drilling date (1 November 2005). The surface layer does not show a microalgae layer, which suggests that during the core drilling the microalgae did not yet have adequate living conditions on the glacier surface. This agrees with Reference Yoshimura, Kohshima and OhtaniYoshimura and others (1997, Reference Yoshimura, Kohshima, Takeuchi, Seko and Fujita2000) who indicated that microalgal growth in the Himalaya starts at the beginning of summer and continues throughout the season. Neither are protozoa observed in this surface layer, probably for the same reason as for the microalgae.
A Podocarpaceae pollen signal is observed at the bottom of the core, between 10.2 and 10.4 m. This suggests pollen dispersion within this layer, interpreted as corresponding to the flowering season (spring–summer), which terminates at 8.2 m (late summer–early autumn) according to the micro-algae profile.
Fluctuations in δD are observed within the upper 5 m section of the core (Fig. 5h). Based on the biological profiles, the layer from 1 to 5.4 m is interpreted as corresponding to the accumulation period autumn–winter 2005. In fact this layer preserves variations in δD signals. Below 5 m the fluctuations in δD are strongly damped, suggesting an important effect due to water percolation, consistent not only with a maximum percentage of ice lenses at 6 m, but also with an algae and protozoa maximum at the same depth. Hence, the layer between 5.4 and 7.2 m is interpreted as corresponding to summer 2004/05. Consequently, the δD record cannot be used to date firn layers of previous years.
Ice layers are almost absent at 1.8–4.6 m and 7.6–8.4 m (Fig. 5e), indicating reduced melt and refreeze. These horizons are interpreted as corresponding to autumn–winter as suggested by the biological profiles. Contrastingly, a high percentage of ice layers are observed within the horizons interpreted as spring 2005, summer 2004/05 and summer 2003/04 (Fig. 5e), suggesting relevant melt–freeze periods during the melt season in this area. The density profile (Fig. 5f) shows an increase from 0.5 g cm−3 to nearly 0.7 g cm−3 at depth, with values approaching the ice density according to the presence of ice lenses. The firn–ice transition was not reached and must occur below 10.4 m.
Fluctuations in Cl−, Na+, K+ and SO4 2− concentrations show near-zero values in layers interpreted as summer 2004/05 and summer 2003/04, and high values in layers interpreted as autumn–winter 2005 and autumn–winter 2004 (Fig. 5; Table 1). High levels of Cl−, Na+, K+ and SO4 2− are also observed in the surface layer corresponding to spring 2005, suggesting that the dampening of chemical signals during summer is due to post–depositional processes, probably related to partial meltwater percolation. The high surface levels observed during drilling suggest that melt and percolation effects are still insignificant.
Table 1. Median concentrations (ppb) of chloride, sodium, potassium and sulphate in winter and summer snow for the Volcán Osorno core samples, along with the summer/winter ratio.
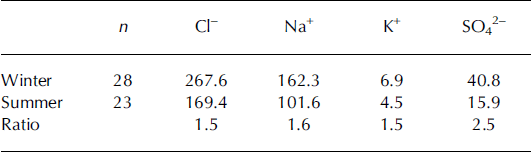
Concerning Ca2+ and Mg2+, a maximum value corresponding to autumn–winter 2004 is observed, which does not occur in the autumn–winter 2005 layer. NO3 − and NH4 + show maximum values in the upper layer corresponding to spring 2005, while seasonal variations are absent below this layer, suggesting that percolation or other post-depositional processes are relevant.
The site shows dominant concentrations of Cl− and Na+ (Table 2), pointing to a dominance of natural constituents, which frequently correspond to sea spray (Reference OlivierOlivier and others 2003; Reference Eichler, Schwikowski, Furger, Schotterer and GäggelerEichler and others, 2004). Concentrations of ions of anthropogenic origin (e.g. NO3 −, NH4 + and SO4 2−)are even lower here than pre-industrial values from cores of the European Alps (Reference Schwikowski, Döscher, Gäggeler and SchottererSchwikowski and others, 1999a) (Table 2).
Table 2. Ion median concentrations of all samples in the Volcán Osorno core

Sea-salt aerosol contribution of chemical species is important at this site, as demonstrated by: (1) the high correlation coefficient between Cl− and Na+ (r = 0.998; Table 3); (2) the proximity of the Cl−/Na+ proportion, 1.11 ± 0.7, obtained in this study (Fig. 5g; Table 2) to the marine reference value of 1.16 (Reference Keene, Pszenny, Galloway and HawleyKeene and others 1986); and (3) the fact that other chemical species that are sea-salt constituents (e.g. Cl−, Ca2+, Na+, Mg2+, K+ and SO4 2− (Reference WatanabeWatanabe and others, 2003)) show high correlation coefficients at this site (Table 3). This finding is not surprising since the drilling area is ∼100 km east of the Pacific Ocean, under the influence of the predominant westerly circulation.
Table 3. Correlation matrix of the Volcán Osorno chemical species, taken as the logarithmic values of concentrations. The underlined numbers are correlation coefficients larger than 0.8, and the bold numbers are correlation coefficients larger than 0.7

Estimation of mass balance
Firn density data indicate that this ice core of 10.4 m corresponds to 5.88 mw.e. According to the profiles of micro-algae, protozoa, pollen and chemical species (Fig. 5), it is estimated that the 10.4 m core includes one annual layer from 2004/05 and a layer from the last accumulation period of autumn–winter 2005. The net mass balance from autumn 2004 to autumn 2005 is 1.51 m w.e., and the winter balance is 2.4 m w.e. Considering a rainfall amount of 1189 mm between 1 April and 31 October 2005 (personal communication from J. Carrasco, 2007) for the coastal station Puerto Montt (∼100 km southwest of the drilling site), our autumn–winter mass-balance estimation shows that the Volcán Osorno accumulation preserved in mid-spring 2005 was twice as much as the autumn–winter–early-spring precipitation recorded at Puerto Montt, which is reasonable considering the strong orographic effect associated with the westerly airflow across the southern Andes. Available data for the mountain station Punta Huano (200 m a.s.l.; located 75 km northeast of Puerto Montt and 70 km southwest of Volcán Osorno) show an annual precipitation of 3095 mm (personal communication from J. Carrasco, 2007). In contrast, the annual accumulation preserved in 2004/05 in the Volcán Osorno core is similar to the annual precipitation of 1621 mm recorded at Puerto Montt between April 2004 and March 2005 (personal communication from J. Carrasco, 2007), which can be explained by the strong melt that is responsible for the ablation of an important autumn–winter–early-spring layer during summer.
Volcán Mocho-Choshuenco summit core analysis
Variations in the biological profile, physical properties, δD and ion chemistry
Similar to the case of the Volcán Osorno core, the biological species preserved in the Mocho-Choshuenco summit core also show variations which are interpreted as seasonal markers (Fig. 6). The microalgae and protozoa peaks show four layers at 5.4–6.4, 6.8–7.4, 7.6–8.4 and 8.6–9.2 m which are interpreted as summer melt seasons. A microalgae peak observed between 4.2 and 4.4 m, which shows a smaller biovolumethan in the other layers, is inferred not to represent a summer melt season, due to protozoa and pollen absence and lack of correlation with chemical and deuterium profiles (Fig. 6). One possibility is that this weak peak is due to wind transport (Reference Müller, Leya and FuhrMüller and others, 2001) from microalgae sources located in nearby (∼30–60 km) lake sites.

Fig. 6. Same as Figure 5, but for the Volcán Mocho-Choshuenco summit core.
In both pollen profiles, a signal is observed in the surface layer between 0 and 1.5 m which is interpreted as corresponding to spring 2005. The absence of microalgae and protozoa in this period agrees with data from the Volcán Osorno core and with the observation by Reference Yoshimura, Kohshima and OhtaniYoshimura and others (1997, Reference Yoshimura, Kohshima, Takeuchi, Seko and Fujita2000) concerning the initial date of algal bloom.
When comparing the microorganism data with those from Podocarpaceae records, three layers interpreted as corresponding to the summer melt season are observed to coincide, with two of those layers coinciding with Nothofagus spp. horizons. Similar to the case of Volcán Osorno, the absence of pollen layers does not necessarily indicate absence of melt, so the information provided by pollen is regarded as confirming the interpretation of the microorganism record.
Two horizons without ice layers are recognized: 0–5.4 m and 5.6–6.8 m (Fig. 6e). When comparing these depths to the biological profiles, the first horizon below 1.5 m is interpreted as corresponding to autumn–winter, with no microorganisms or pollen. In contrast, the 5.6–6.8 m horizon coincides with a layer of microalgae, protozoa and pollen, which is located between two layers with 100% ice. The ice layer at 5.4 m shows a sudden density increase from 0.55 to 0.77 g cm−3, (Fig. 6f) and an accompanying dampening of the deuterium signal. This suggests that a strong percolation has occurred at 5.4 m which is responsible for obliterating the isotope record (Reference Schwikowski, Brütsch, Casassa and RiveraSchwikowski and others, 2006).
All the chemical species (Cl−, Na+, Mg2+,K+, SO4 2−, NO3 − and NH4 +) show fluctuations throughout their profiles (Fig. 6). Nevertheless, there is no obvious correlation of these fluctuations with seasonal layers interpreted from biological profiles. What is clear is that all chemical species exhibit concentrations that tend to zero at the deepest layers (9.2–10.4 m), suggesting that meltwater percolation is responsible for destroying the signals at depth. In the case of the periods corresponding to autumn–winter 2002–03, a maximum signal of Na+, Mg2+, K+ and SO4 2− concentrations is observed, with a minimum in the summers of 2002/03 and 2003/04, similar to the case of Volcán Osorno. However, this is not true for summer 2004/05, autumn–winter 2004 and summer 2001/02, possibly because of strong percolation that can cause a migration of chemical species to deeper layers and in this manner obliterate the seasonal signal. The relevance of the melt process is illustrated by the thin layers which are seen to correspond to the periods interpreted as summer.
Main atmospheric sources of chemical trace species
Similar to the case of Volcán Osorno, the site is characterized by the dominance of natural constituents, indicated by the Cl− and Na+ concentrations recorded throughout the core (Table 4). Sea-spray contribution of chemical species is important at this site, as demonstrated by (1) the high correlation coefficient between Cl− and Na+ (r = 0.90; Table 5); (2) the proximity of the Cl−/Na+ ratio (Fig. 6g) to the marine reference value of 1.16 (Reference Keene, Pszenny, Galloway and HawleyKeene and others 1986; Reference Schwikowski, Brütsch, Gäggeler and SchottererSchwikowski and others 1999b) in the upper 6 m. Below 6.6 m the Cl−/Na+ ratio deviates substantially from the marine reference value, which suggests a strong elution due to strong percolation at this site (Reference Davies, Vincent and BrimblecombeDavies and others, 1982; Reference ShiraiwaShiraiwa and others, 2002); and (3) the fact that other related sea-salt constituents such as Cl−, Na+, Ca2+ and Mg2+ (Table 5) show high correlation coefficients at this site (Reference WatanabeWatanabe and others, 2003). As in the Volcán Osorno ice core, the marine origin can be explained by the proximity of the site to the Pacific Ocean (∼100 km) and the prevailing westerly circulation.
Table 4. Ion median concentrations of all samples in the Volcán Mocho-Choshuenco summit core

Table 5. Correlation matrix of the chemical species from the Volcán Mocho–Choshuenco summit core. For more details see Table 3

Estimation of mass balance
Density data indicate that this 10.2 m long core is equivalent to 6.16 m w.e. According to the analysis of biological profiles in combination with the ice-layer information and deuterium, it is estimated that three annual layers are preserved (2002/03–2003/04–2004/05), in addition to the last accumulation period (winter–autumn 2005). The net mass balance is 0.66 m w.e. for 2002/03, 0.56 m w.e. for 2003/04, and 0.86 m w.e. for 2004/05, with a mean annual (early-autumn to early-autumn) net mass balance from 2003 to 2005 of 0.69 ± 0.15 m w.e. for this site. The winter 2005 balance is 2.12 m w.e.
In 2003 a mass-balance programme was initiated on the southeastern glacier of Volcán Mocho-Choshuenco, calculating the net mass balance for the period 2003/04 based on the stake method (Reference Rivera, Bown, Casassa, Acuña and ClaveroRivera and others, 2005). The summit stake was frequently lost due to strong winds, with the closest stake (No. 21) being located at 2169 m a.s.l. At stake 21 the annual mass balance was 0.9 m w.e. and the winter balance was 2.4 m w.e. (Reference Rivera, Bown, Casassa, Acuña and ClaveroRivera and others, 2005), in good agreement with the mass-balance values of 0.69 m w.e. and 2.12 m w.e. estimated from the summit core for 2005. These results demonstrate the robustness of the multi-proxy dating method including biological, physical and chemical parameters as presented in this study.
Volcán Mocho-Choshuenco 2000 m a.s.l. core analysis
One firn layer of 8.4 m depth was observed at this site, which is interpreted as corresponding to autumn–winter 2005. An ice layer was detected (Fig. 7) below the firn down to the bottom of the core (10 m). Therefore the 8.4 m depth corresponds to the firn–ice interface. During the drilling (26 October 2005), the firn was saturated with water at the firn–ice interface. A water-saturated layer at the firn–ice transition has been commonly found at other temperate glaciers such as Glaciar San Rafael (Reference YamadaYamada, 1987) and Glaciar Tyndall (Reference ShiraiwaShirawa and others, 2002) in Patagonia.

Fig. 7. Profiles of the ice core from Volcán Mocho-Choshuenco at 2000 ma.s.l.: (a) algal biovolume; (b) protozoa abundance; (c) Podocarpacea pollen concentration; (d) Nothofagus spp. pollen concentration; (e) copepod abundance; (f) nauplius (larval stage of copepod); (g) ice-layer percentage; (h) firn density (vertical line corresponds to the firn/ice transition value of 0.83 g cm−3 (Reference PatersonPaterson, 1994)); and (i) deuterium. The lower grey shaded area indicates the lower ice layer, estimated to correspond to the 2004/05 summer period (S) and older ice. The upper grey shaded area indicates the pollen flowering period (spring: Sp) estimated from pollen peaks. The autumn–winter period (in white) is labelled A-W.
The δD values are observed to fluctuate in the firn layer (Fig. 7), with a dampening of the signal below that depth. This indicates that the winter–autumn 2005 signal is still preserved, whereas the signal at depth, corresponding to summer 2004/05, is obliterated by meltwater percolation. Microalgal biovolume and pollen concentrations are very small at Volcán Mocho-Choshuenco at 2000 m a.s.l. compared to the values found in the cores drilled at the summits of Mocho-Choshuenco and Volcán Osorno, and are negligible in the ice layer corresponding to summer 2004/05. These biological components are believed to be washed out by strong melt and percolation. It is suggested that the algal biovolume values in this core originate from algal transportation by wind from their source during autumn–winter. In contrast, protozoa, copepods and nauplius are preserved within the summer 2004/05 ice layer (Fig. 7). A surface signal between 0 and 2.8 m is observed in the Podocarpaceae pollen profile, which is interpreted as corresponding to spring 2005.
Estimation of mass balance
Density profile data indicate that the 10.0 m long core corresponds to 6.0 mw.e. According to the profiles of protozoa, copepod, nauplius, pollen, ice layers and deuterium, the core is interpreted as including a 2.95 m w.e. layer from the last accumulation period (autumn–winter) and an underlying ice layer with no indication of an older annual or seasonal transition. The 2.95 m w.e. winter balance is in good agreement with the winter balance of 2.9 mw.e. estimated by Reference Rivera, Bown, Casassa, Acuña and ClaveroRivera and others (2005) from stake measurements made in 2003 at the same site (stake 18).
Conclusions
In all cores, seasonal cycles in deuterium are dampened substantially by meltwater percolation below the last autumn–winter accumulation layer. This is consistent with a sudden increase in firn density and also with the presence of ice layers within the cores.
The Cl−/Na+ ratio and the high correlation of sea-salt constituents indicate that marine contribution is the main source of these species. This is reasonable considering the proximity of the core sites (∼100 km) to the Pacific Ocean and the prevailing westerly circulation. At the highest site (Volcán Osorno), some chemical species (Cl−, K+, Na+ and SO4 2−)show seasonal variations. Based on the large concentration values of these species found within the surface layer (spring 2005) and the presence of abundant ice layers within the summer snow and firn, this suggests that partial percolation is the most probable cause for seasonality. Other species at Volcán Osorno (NO3 − and NH4 +) show no seasonal variations, suggesting that they are more affected by melt and percolation which obliterates the signal. At the summit of Volcán Mocho-Choshuenco, the chemical species show no clear seasonality, indicating that melt and percolation are more relevant at this site.
Microalgae, protozoa, copepod and pollen show clear seasonal cycles in the summit cores. Maximum values of microorganisms are interpreted as corresponding to the summer melt season, when the presence of meltwater favours their growth. Maximum levels of pollen occur in the spring, which corresponds to the flowering period of tree species in the surrounding Valdivian forest at lower altitudes. At the lowest site, Volcán Mocho-Choshuenco (2000 m), the microalgae values are very small throughout the core. Within the ice layer at depth, microalgae and pollen records do not show maximum values as might be expected from the data observed in the summit cores, suggesting that they are strongly affected by melt and percolation, as evidenced by a water table detected at 8.4 m depth at the firn–ice transition.
From the biological, physical and chemical records, the following interpretations are derived: the core from Volcán Osorno presents an annual layer (2004/05) and a layer from the last accumulation period (winter–autumn 2005); the core from Volcán Mocho-Choshuenco drilled at the summit presents three annual layers (2002/03–2003/04–2004/05), plus a layer from the last accumulation period (winter–autumn 2005). The core from Volcán Mocho-Choshuenco drilled at 2000 m a.s.l. only presents one layer, corresponding to the last accumulation period (winter–autumn 2005). Winter and net mass-balance estimations of ice cores from Volcán Mocho-Choshuenco are in good agreement with values previously obtained by means of the stake method in the period 2003/04 (Reference Rivera, Bown, Casassa, Acuña and ClaveroRivera and others, 2005).
The microorganisms and pollen are therefore useful seasonal markers for ice-core dating and reconstruction of past annual mass balance in temperate glaciers located in the Chilean lake district. This is especially important considering that at these sites stable-isotope and chemical-species records are destroyed by meltwater percolation.
Acknowledgements
This work was supported by Fondo Nacional de Ciencia y Tecnología of Chile (FONDECYT 1040989 and 1061269) and Centro de Estudios Científicos (CECS). The CECS is funded by the Chilean Government through the Millennium Science Initiative and the Centers of Excellence Base Financing Program of CONICYT (Comisión Nacional de Investigación Cientifica y Technológica de Chile). CECS is also supported by a group of private companies which at present includes Antofagasta Minerals, Arauco, Empresas CMPC, Indura, Naviera Ultragas and Telefónica del Sur. We acknowledge field support from G. Campos, D. Schwerer, R. Mella, M. Rodríguez and M. Arévalo. We thank R. Silva (Universidad Austral) for the scanning electron microscope imagery, P. Moreno (Universidad de Chile) for advice on pollen identification, and R. Jorquera and H. Silva for collaboration at CECS.
















