Symbols
c 0 mass of solution introduced
E(x) relative error in parameter x
F liquid-water content, liquid volume divided by total volume
g acceleration due to gravity
g′ acceleration due to centrifuging
k intrinsic permeability of snow
L equivalent length of centrifuged sample
L′ length of centrifuged sample
m o molal concentration of sodium hydroxide
m s mass of snow sample
r mean radius of centrifuge
S m mobile water, S w—S w1
S w water saturation, liquid volume divided by pore volume
S wi irreducible water saturation
S ★ effective water saturation, (S w—S wi)/(1—S wi)
t equivalent time for a sample drained by gravity
t′ time of centrifuging
T temperature
u flux of water, flow through a unit area per unit time
v a, v i, v s, v w volumes of air, ice, sample, and liquid
α 5.47 × 106 m−1 s−1
β molal temperature depression constant of the dissolved substance
ρ i, ρ s, ρ w densities of ice, snow, and water
ϕ porosity, pore volume divided by total volume
ω angular velocity of centrifuge
1. Introduction
There are many reasons for wanting to know the liquid-water saturation and porosity of snow. Every investigation of the snow cover uses information such as the snow density, state of metamorphism, liquid-water storage capacity, and/or liquid-water transmission rate. Furthermore, virtually all of the important material properties of snow are related to its density and history of liquid saturation. Two of the most important pieces of information about snow, either on the scale of kilometers or millimeters, are water saturation and porosity. These two pieces of information are necessary for even a crude approximation of such varied properties as snow strength and water flow rates. Accordingly, much attention has been given to developing devices for determining the liquid-water content and density of snow. While some of these devices work rather well (see Table I), others can be shown to be unacceptable conceptually. Because of the widespread use of devices which fall into the latter category, all of the popular devices are analyzed here in order to identify their limitations.
Table I. Typical calculation errors when E(S wi) = E(v s) = E(m s) = 0
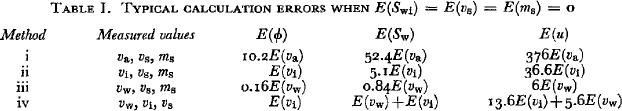
Snow hydrologists have been concerned principally with the "free-water content F" of snow, i.e. the fraction of the total volume occupied by the liquid phase. For most purposes it is more meaningful to separate the free-water content into its two component parameters, the liquid-water saturation S w and porosity ϕ. These three are related by
Since it is necessary to determine both S w and ϕ for virtually every wet-snow problem, F can be calculated from known values of S w and ϕ if desired.
Neglecting the weight of the air phase,
relates the snow density ρ s to the ice mass per unit sample volume plus the liquid mass per unit sample volume. Thus the snow density can also be calculated directly from known values of S w and ϕ. The advantage of using S w instead of F can best be illustrated by the flux–concentration relationship for water (Reference Colbeck and DavidsonColbeck and Davidson, 1973),
where
S wi is the irreducible water content which cannot be removed by gravity drainage, α is a constant, the intrinsic permeability k is a function of only the porous matrix, and S ★ is a function of only the mobile liquid fraction in the pore volume.
2. Calculation errors for S w and ϕ
There are four fundamental equations; two describe the mass and volume balances and two are definitions (neglecting the air mass):
These four equations contain seven variables hence three pieces of information must be supplied. Usually the sample mass and volume are determined independently, in which case either the air, water, or ice volume must be measured directly. If the sample mass or volume is not determined independently, then two other quantities would have to be measured. The errors inherent in four of the ten possible combinations of the volume and mass variables are analyzed here. First, the three possible cases which include v s and m s are considered. Then, the optimum combination for in situ sampling—v s, v i and v w—is analyzed:
(i) Measure v a directly
This procedure would be attractive for a destructive sampling technique because phase changes during sample preparation would not matter except that the solubility of air in water would have to be considered. Unfortunately, in order to calculate S w and ϕ, it is necessary to divide by the difference between two large numbers, a procedure which inevitably leads to large errors. Using the definition of relative error,
and assuming no error in the measurements of m s and v s,
and
For a typical case where v i = 500 × 10−6 m3, v w = 75 × 10−6 m3, m s = 0.533 kg, v s = 10−3 m3 and v a = 425 × 10−6 m3,
and
These large values render this approach useless unless extremely precise measurements of v a are possible. It is important to note that E(S w) increases rapidly as S w approaches S wi.
(ii) Measure v i directly
While this method is widely used because of its simplicity, large errors are associated with calculating S w from the measured quantities. Again assuming no error in the measurements of m s and v s,
and
Note that the liquid saturation only enters this equation through the denominator, m s—v i ρ i. As the liquid saturation decreases, m s—v i ρ i decreases and a large error occurs in the calculation.
For the sample case,
and
but E(S w) is much higher at lower values of saturation. As shown on Figure 1, E(S w)/E(v i) approaches negative infinity as S w approaches zero.

Fig. 1 The ratios of error in calculating Sw to the error in measuring vi and vw are shown for methods (ii) and (iii) respectively (assuming E(ms) = E(vs) = 0, vs = 10−3 m3 and vi = 0.5 × 10−3 m3). For method (ii) the ratio is very large for all values of Sw and approaches negative infinity as Sw vanishes. For method (iii), however, the ratio reaches an upper limit of unity as Sw vanishes and is rather insensitive to the value of Sw over the common range of saturations.
While this method may be useful for calculating the snow porosity, it will inevitably lead to large errors in the calculated value of water saturation unless very precise measurements of v i are possible. As shown later, if this method were used to calculate or infer variations in the flow field of liquid water, a great deal of uncertainty would be associated with the results.
(iii) Measure v w directly
This is the principle of many common devices and, since the error analysis shows that this method is most likely to produce consistent and accurate results, it is analyzed in more detail. We find
and
where the inequality sign is used to show that we only know the upper limit of the error. Unlike the two previous methods, as the volume of water approaches zero, the error in S w approaches a small upper limit instead of becoming infinite (see Fig. 1). For the sample case,
and
From these considerations it is clear that the direct measurement of the liquid is inherently a more accurate method of determining both S w and ϕ (see Table 1). The use of methods (i) or (ii) could only be justified if it could be shown that the error in measuring the volume of water directly was much greater than the error in measuring the volumes of ice or air. However, as shown later, the dielectric constant is very sensitive to the liquid volume, hence an accurate determination of S w is possible.
For method (iii) the errors in calculating ϕ or S w are more sensitive to the errors of measurement of m s and v s than of v w. As shown by Equations (18) and (19), E(ϕ) and E(S w) increase inversely with the quantity ρ i v s ϕ. For a given situation, the errors in S w and ϕ decrease as sample size increases, thus demonstrating the importance of avoiding small samples.
(iv) Measure v w, v i and v s directly
The use of a snow sampling kit to measure m s and v s is inherently undesirable because it is frequently necessary to use two samples to get one calculated value of S w or ϕ. Direct measurements of v w, v i and v s are most desirable. In principle these measurements are possible, for example, by an electromagnetic instrument which senses the volumes of solid and liquid in a volume of sample which is predetermined by the nature of the instrument. The errors in calculating S w and ϕ are given by
and
For the sample case these errors are
and
If the predetermined volume being sensed v s is known accurately, this method should work well for a remote-sensing system which can accurately distinguish between the liquid and solid phases.
3. Calculation errors for u
The liquid-water content is most often measured to infer or calculate variations in the flow field of water. Because of the sensitivity of water flux to water saturation as shown by
any error in the determination of S ★ will be magnified in the calculation of u. The related error in flux is bounded by
Accordingly, errors inherent in the device used to determine S w must be avoided as much as possible. The error in calculating flux could be significantly reduced by taking a large number of samples in order to determine S w more accurately. This procedure is difficult to follow in practice, however, since destructive sampling procedures are inherently undesirable and in situ instrumentation is rarely used in multiple arrays at each level in a snow-pack. The question of lateral variability must also be addressed if multiple samples are to be taken from a single snow layer.
At worst E(u) increases directly as E(k) but, since direct measurements of intrinsic permeability are rarely made, k must be calculated from determinations of ϕ and the average grain size (e.g. Reference ShimizuShimizu, 1970). From data given by Reference KuroiwaKuroiwa (1968), it can be shown that k increases as exp (15.9ϕ), which is a typical permeability–porosity relationship for a porous medium. Neglecting any error in the determination of the average grain size, the relative error in permeability is highly sensitive to E(ϕ) as shown by
Again, the need to determine ϕ accurately is apparent.
From %Equation (4),
where the "mobile-water saturation" is given by
As a consequence, E(S ★) is most sensitive to the measurement of S m since S wi/(1—S wi) is a small number. When the error in S ★ is expressed in this way, the advantages of using a system which measures only the mobile water are apparent. This result suggests that for the purpose of calculating the flux of water, a controlled capillary withdrawal of the mobile liquid might be better than measurement of the total liquid saturation. For the determination of the material properties, however, the total liquid saturation is required.
E(S ★) can also be expressed as
which shows that E(S ★) becomes very large as S w approaches S wi. Apparently, the error in calculating flux from measurements of S w becomes very large as flux becomes vanishingly small.
From direct determinations of S w, S wi and ϕ, the error in calculating the flux of water is bounded by
The upper bound of E(u) is approximated at large fluxes by
and the error increases with decreasing flow rates. Again, the need to determine the liquid content accurately is apparent. Likewise, the need to make an accurate porosity determination is shown.
If method (ii) is used to determine the liquid saturation, even in the absence of errors in the measurements of m s, v s or S wi, for large flow rates
and E(u) increases rapidly as u decreases.
This large error in the calculated value of flux precludes the possibility of getting a meaningful estimate of the flow field from measurements of the ice volume and explains why reproducible results have not been generally obtained with a melting calorimeter.
If method (iii) is used and the volume of liquid is sensed directly, at large flow rates
Even in the absence of error in measuring v s, m s or S wi, the error in calculating the flux of water from the measured volume of water is six times greater than the error in the measurement itself. Assuming equal errors in the measurements of v w and v i, the advantage of working directly with the volume of water is apparent. It is important, however, that the volume of ice is generally about six times greater than the volume of liquid hence its error of measurement might be somewhat lower. These errors must be considered, for example, in choosing between the melting and freezing calorimeters.
If method (iv) is used with a remote-sensing system, the error in calculating flux would be
Clearly this method compares favorably with any of the others although large errors could still occur in the calculation unless very accurate measurements are possible.
4. Destructive sampling techniques
With this background, a systematic discussion of the various types of "saturometers" can be made. Excluding those which cannot give accurate results a priori, we consider two types—those which must take a sample from the snow cover and those which can get the necessary information in situ.
4a. Freezing calorimeter
Reference Radok, Radok, Stephens and SutherlandRadok and others (1961) discussed the inherent advantage of the freezing calorimeter. They point out that in calculating "snow quality" from measurements with a melting calorimeter, it is necessary to take the small difference between large numbers, hence large errors are possible. In the freezing calorimeter, however, the calculation is different and the errors are not likely to be so large. This conclusion is identical to that derived above for techniques which measure v w versus those which measure v i.
Reference LeafLeaf (1966) reports achieving an accuracy of 1% liquid by weight or ![]() by volume with this technique. This suggests that S
w could be calculated to within an accuracy of 1%, a feat which would only be possible if there were essentially no errors in measuring v
s and m
s and if v
w was calculated to within 1% from the calorimetry data. The latter seems unlikely in view of the difficulty of handling the snow samples without causing any phase changes, but nevertheless this method is promising and hopefully further refinements will be made. The inherent advantage of a direct measurement of v
w probably compensates for the fact that freezing is more difficult than melting. Perhaps this method's major disadvantage is the fact that it is a destructive sampling technique, hence some disturbance of the flow field will necessarily be associated with its use.
by volume with this technique. This suggests that S
w could be calculated to within an accuracy of 1%, a feat which would only be possible if there were essentially no errors in measuring v
s and m
s and if v
w was calculated to within 1% from the calorimetry data. The latter seems unlikely in view of the difficulty of handling the snow samples without causing any phase changes, but nevertheless this method is promising and hopefully further refinements will be made. The inherent advantage of a direct measurement of v
w probably compensates for the fact that freezing is more difficult than melting. Perhaps this method's major disadvantage is the fact that it is a destructive sampling technique, hence some disturbance of the flow field will necessarily be associated with its use.
4b. Centrifuges
Much attention has been given to the use of centrifuges in soil physics, petroleum reservoir engineering, and snow hydrology. The attractive feature of a centrifuge is that large accelerative forces are exerted on the fluids, thereby decreasing the time necessary to drain a porous sample. Reference StallmanStallman (1964) shows that
and
where primed length L′, time t′ and acceleration g′ are for the centrifuge's frame of reference and unprimed L and t are their equivalents in a sample subjected only to Earth's gravity g. When g′ = 1 000g, a 10 mm long sample centrifuged for 40 min is equivalent to the drainage of a 10 m column for 76 years provided the bottom of the sample remains in contact with the liquid during the 40 min of centrifuging. Unfortunately these scaling laws do not apply to the centrifuges currently in use by snow hydrologists because the samples do not remain in contact with the extracted liquid so there is some uncertainty about how the snow centrifuge should be scaled. Furthermore, Reference Slobod, Slobod, Chambers and PrehnSlobod and others (1951) state that displacement occurs only down to the "connate value" or irreducible water saturation. If Slobod and others are correct, only the mobile water can be extracted by this method and the total liquid-water content cannot be found by centrifuging! This does not necessarily rule out the use of centrifuges since, even if only the mobile component of the water were removed and measured, this could be useful information. It must be noted, however, that centrifuging snow samples at 60 revolutions per second can cause some consolidation. The time of consolidation effectively increases by a factor of (g′/g)2 (Reference Terzaghi and MeinzerTerzaghi, 1942).
Reference Terzaghi and MeinzerTerzaghi (1942) described centrifuges by noting that, if the accelerative force is increased N times, the body forces are increased to NS w per unit volume while the retentive forces remain constant. Therefore the height of capillary rise is decreased to N −1 h c, hence the capillary end effect is reduced. Terzaghi also states that this scaling is invalid when the water is discontinuous because the weight of the particles becomes small enough that the surface tension can balance it. Contrary to Slobod and others, Terzaghi believes that the discontinuous moisture in centrifuging is less than the discontinuous moisture in gravity drainage, hence more than just the mobile water is extracted. Unfortunately, there are different interpretations of just how much liquid can be extracted by centrifuging. This question is more important for snow than for most other porous materials because of the limitations placed on the centrifuging of snow by the problems of melting and compacting during the process. To provide some insight into the question of how much liquid can be extracted by centrifuging, samples of hydrophilic glass beads were soaked with water and centrifuged under conditions similar to those possible in a field situation. Although glass beads were used in place of snow in these tests, the results are applicable to snow because all of the important parameters which affect the retention of water in snow were closely simulated in these tests. The samples were well soaked to ensure complete wetting and then hand-centrifuged for 60 s at about 7 revolutions per second to reduce their liquid content to about that of a freely draining snow cover. The samples were then centrifuged by a machine for 60 s at 33.3 revolutions per second plus the 30 s necessary to accelerate and decelerate the machine. The residual water present following centrifuging, as determined by oven-drying the samples, was highly dependent on the size of the glass beads (see Fig. 2) over the range of grain sizes common in natural snow covers

Fig. 2 The residual water saturation left behind after centrifuging decreases with increasing particle size. Much uncertainly is connected with the use of centrifuges because of this residual water.
(Reference WakahamaWakahama, 1968). This result shows that different amounts of water will be extracted from different parts of a snow pack due to the occurrence of different grain sizes. Therefore, even as a relative measure of the amount of water present and the amount of water flowing, the centrifuge can give misleading results.
In the past Reference Yosida, Yoshida and ŌuraYosida (1967) and Reference LaChapelleLaChapelle (1956) have noted the partial retention of water by snow samples during centrifuging. Hopefully, the results given here will discourage the widespread use of centrifuges in snow hydrology. Centrifuges have often been used as a measure of the spatial variability of the flow field of water in snow (e.g. Reference Langham, Santeford and SmithLangham, 1974) but as shown by Equation (12), the error in flow is six times as large as the error in S w. Unfortunately, the error in S w as determined by centrifuging is affected by such things as grain size, melting, compaction, and time and rate of spinning. Under any circumstances, some water will be retained by the snow sample, a fact which precludes the quantitative use of the information obtained from a centrifuge. Reference LaChapelleLaChapelle (1956) showed that some qualitative information can be obtained with a centrifuge, but the use of centrifuges for purposes such as determining the spatial and temporal variability of the flow field in normal snow covers is risky. Much of the infered non-uniformity in the flow field is simply due to the inherent limitations of the centrifuge.
4c. Solution method
Reference BaderBader (1948) proposed a simple method in which a dilute solution of sodium hydroxide is added to a known quantity of wet snow and the temperature depression is measured. As long as the temperature depression is small (≈ 1.5 deg) and no significant errors occur in measuring the solution weight or sample weight, an accurate determination of the free water content F can be made. The relative error is
or, for a typical case described by Reference BaderBader (1950),
Thus Bader's method offers a quick and easy alternative to calorimetry. Assuming accurate weight, volume and temperature measurements, useful information on the free water content should be obtainable. Probably the biggest error in this method would be introduced by inaccurate measurements of the molal concentration m 0 of the solution of sodium hydroxide. Neglecting other errors,
or, for a typical case,
This shows that the solution must be prepared carefully.
5. Non-destructive measuring devices
While some of these destructive sampling techniques give reasonably accurate information about the liquid water in a sample, they all suffer the serious disadvantage of disturbing the flow field by the act of removing the sample. This fact precludes repeated sampling to determine the temporal variations at a point in a snow cover. Repeated sampling to determine the local spatial variations is also suspect because of disturbances to the flow field caused by the creation of new surfaces within the flow field. Accordingly, the use of in situ or remote-sensing devices is necessary to obtain the most useful information, and several such devices are reviewed here. Other possible methods, which have not yet been applied to snow, include nuclear magnetic resonance (NMR), time-domain reflectometry, Raman scattering (see Reference MillerMiller, 1972), and acoustic methods.
5a. Dielectric devices
The large contrast between the dielectric constants of liquid water and ice at megahertz frequencies has provided the basis for measuring the liquid-water content of various materials at least since the early 1930's when meters were used to determine the liquid content of wheat. Perhaps Reference GerdelGerdel (1954) was the first to apply these devices to snow using a capacitance probe operating at a frequency of 1.5 MHz. Although the dielectric constant of snow is very sensitive to small changes in the volume of liquid water present, it is difficult to make a strict interpretation of the dielectric constant of the solid-liquid-gaseous mixture because of the importance of shape factors on the contribution of each phase to the dielectric constant of the mixture.
Reference Ambach and DenothAmbach and Denoth (1975) have recently improved the capacitance probes used in snow by designing an instrument which operates at frequencies up to 20 MHz. At higher frequencies, the effect of grain size is minimized but the snow density must be known to calculate the free water content from the dielectric constant (Reference Ambach and DenothAmbach and Denoth, 1975). The need to measure the snow density, a distinct disadvantage of this approach, arises because the mixing formulae (used to account for the shape factors) require the use of the value of the dielectric constant extrapolated to an infinite frequency. The dielectric constant then has only a real part, hence the density must be determined separately in order to supply enough information to calculate both the porosity and liquid saturation. For the purposes of designing an in situ instrument, the lack of information about the imaginary part of the dielectric constant is very unfortunate. Nevertheless, the dielectric devices are useful instruments with a standard error of about 0.5% by volume (Reference ThomasThomas, 1966; personal communication from A. Denoth).
At microwave frequencies, the real and imaginary parts of the dielectric constant can be determined simultaneously, thus providing all of the information necessary to calculate the porosity and water saturation in a known volume of wet snow (Reference Sweeny and ColbeckSweeny and Colbeck, 1974). Unfortunately, the sophisticated microwave equipment is not easily adapted to field situations, so dielectric measurement by capacitance (Reference Ambach and DenothAmbach and Denoth, 1975) is probably the best available at this time. Perhaps the best hope for developing a system to obtain information about porosity and water saturation in wet snow is the active microwave system. Reference Linlor, Linlor, Meier, Smith, Santeford and SmithLinlor and others (1974) describe the application of such a system for obtaining profiles of snow wetness. The ultimate system would determine profiles of solid and liquid contents in a snow cover, thus providing information about the degree of layering, propagation of melt-water waves, state of ripening, depth, etc.
5b. Other devices
The soil-water tensiometer of Reference Richards and GardnerRichards and Gardner (1936) measures the negative gage pressure in the liquid phase in an unsaturated soil. In snow, the negative pressure, or "tension", is determined primarily by the liquid-water saturation and grain size. For a thoroughly wetted snow where the grain size is stable but the liquid saturation changes with the flux of water, tension measurements provide a direct indication of the flow rate (Reference ColbeckColbeck, 1976). This correlation between flow rate and tension at a point provides a useful method for observing the flow field without providing any direct knowledge of the porosity or water saturation. For example, Reference WankiewiczWankiewicz (unpublished) used tensiometers to make extensive observations of the flow of water in a deep mountain snow-pack. Unfortunately, tensiometers are difficult to use in a large-grained, porous medium like snow, hence in situ measurements with a capacitance probe may be more successful. Also, capacitance probes are free from the freezing problems of tensiometers; the capacitor can be positioned before the seasonal snow falls and left in position during freeze–thaw cycles.
Methods currently in use to determine the water equivalent of snow include terrestrial gamma-ray surveys (Reference Peck and BissellPeck and Bissell, 1973) and nuclear profiling gages. The use of nuclear sources which are attenuated by the snow was introduced by Reference Gerdel, Gerdel, Hansen and CassidyGerdel and others (1950) and refined by Smith (e.g. Reference Smith, J. L., Willen and OwensSmith and others, 1965). The resolution of these gages is approaching the point of development necessary to detect changes in the flow rate of water. However, even in the absence of any error in ϕ or S wi, Equation (12) shows that E(u) is typically six times as large as E(S w). If at most a ten per cent error in flow rate is desired, the liquid-water saturation would have to be determined to within a relative error of less than two per cent. Typically this means a measurement of total water mass accurate to within two parts per thousand, a feat which is beyond the capability of the nuclear gages currently in use.
6. Conclusions
Liquid-water saturation and porosity are two of the most basic pieces of information about a snow cover. These two parameters largely control such important properties as reflectivity, rheology, and water flow rates. Nevertheless, adequate methods for measuring these properties do not exist and further developments are necessary.
Besides the inherent limitations of destructive sampling techniques, the two most commonly used sampling devices have physical limitations. The centrifuge does not extract all of the liquid, a fact which has been frequently cited in the past. The problem with the centrifuge is that the water left in the sample is a complicated function of structural parameters such as grain size. For example, the water left behind in a sample of glass beads depends on the grain size over the range of sizes commonly observed for snow (see Fig. 2). This fact precludes the use of a centrifuge to infer accurately the flow field in a snow cover. From data taken by the melting calorimeter, both the water saturation and porosity can be calculated. While the calculated value of porosity may be fairly accurate, the calculated value of water saturation is generally highly inaccurate since any error in the measurements is magnified by the nature of the calculation for the liquid-water saturation (see Fig. 1). Accordingly, the use of either the melting calorimeter or centrifuge to determine the spatial or temporal variations in the flow field would be risky. What appear to be variations in flow might just be due to errors inherent in the methods.
Although the response of a tensiometer has been related experimentally to the liquid flow rate in snow, the high-frequency capacitors seem to offer more advantages for making in situ determinations of the liquid-water saturation for research studies. The advantages of the capacitance probes include easy coupling with the snow and a lack of freeze–thaw problems. Eventually efficient methods must be developed for interrogating the snow cover remotely in order to provide the necessary information for hydrological forecasting practices.
Acknowledgements
Drs W. Ambach, G. D. Ashton, and T. E. Osterkamp, have helped prepare this manuscript by suggesting improvements. My support during the period of preparation was provided by Project 4A161102AT24, Research in Snow Mechanics at CRREL.







