Article contents
Nanoparticle diffusion in sheared cellular blood flow
Published online by Cambridge University Press: 24 May 2019
Abstract
Using a multiscale blood flow solver, the complete diffusion tensor of nanoparticles (NPs) in sheared cellular blood flow is calculated over a wide range of shear rate and haematocrit. In the short-time regime, NPs exhibit anomalous dispersive behaviors under high shear and high haematocrit due to the transient elongation and alignment of the red blood cells (RBCs). In the long-time regime, the NP diffusion tensor features high anisotropy. Particularly, there exists a critical shear rate (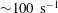 ${\sim}100~\text{s}^{-1}$) around which the shear-rate dependence of the diffusivity tensor changes from linear to nonlinear scale. Above the critical shear rate, the cross-stream diffusivity terms vary sublinearly with shear rate, while the longitudinal term varies superlinearly. The dependence on haematocrit is linear in general except at high shear rates, where a sublinear scale is found for the vorticity term and a quadratic scale for the longitudinal term. Through analysis of the suspension microstructure and numerical experiments, the nonlinear haemorheological dependence of the NP diffusion tensor is attributed to the streamwise elongation and cross-stream contraction of RBCs under high shear, quantified by a capillary number. The RBC size is shown to be the characteristic length scale affecting the RBC-enhanced shear-induced diffusion (RESID), while the NP submicrometre size exhibits negligible influence on the RESID. Based on the observed scaling behaviours, empirical correlations are proposed to bridge the NP diffusion tensor to specific shear rate and haematocrit. The characterized NP diffusion tensor provides a constitutive relation that can lead to more effective continuum models to tackle large-scale NP biotransport applications.
${\sim}100~\text{s}^{-1}$) around which the shear-rate dependence of the diffusivity tensor changes from linear to nonlinear scale. Above the critical shear rate, the cross-stream diffusivity terms vary sublinearly with shear rate, while the longitudinal term varies superlinearly. The dependence on haematocrit is linear in general except at high shear rates, where a sublinear scale is found for the vorticity term and a quadratic scale for the longitudinal term. Through analysis of the suspension microstructure and numerical experiments, the nonlinear haemorheological dependence of the NP diffusion tensor is attributed to the streamwise elongation and cross-stream contraction of RBCs under high shear, quantified by a capillary number. The RBC size is shown to be the characteristic length scale affecting the RBC-enhanced shear-induced diffusion (RESID), while the NP submicrometre size exhibits negligible influence on the RESID. Based on the observed scaling behaviours, empirical correlations are proposed to bridge the NP diffusion tensor to specific shear rate and haematocrit. The characterized NP diffusion tensor provides a constitutive relation that can lead to more effective continuum models to tackle large-scale NP biotransport applications.
- Type
- JFM Papers
- Information
- Copyright
- © 2019 Cambridge University Press
References
Liu Supplementary Movie 1
Instantaneous NP-RBC distribution under unbounded simple shear flow at 100 s-1 shear rate and 40% RBC concentration. Views on the left column show all the NPs and RBCs. Views on the right column show only a few RBCs so as to visualize the NP phase and the single RBC morphology.
Liu Supplementary Movie 2
Instantaneous NP-RBC distribution under unbounded simple shear flow at 2000 s-1 shear rate and 40% RBC concentration. Views on the left column show all the NPs and RBCs. Views on the right column show only a few RBCs so as to visualize the NP phase and the single RBC morphology.
- 24
- Cited by


