Article contents
The instability of gyrotactically trapped cell layers
Published online by Cambridge University Press: 15 April 2019
Abstract
Several metres below the coastal ocean surface there are areas of high ecological activity that contain thin layers of concentrated motile phytoplankton. Gyrotactic trapping has been proposed as a potential mechanism for layer formation of bottom-heavy swimming algae cells, especially in flows where the vorticity varies linearly with depth (Durham et al., Science, vol. 323(5917), 2009, pp. 1067–1070). Using a continuum model for dilute microswimmer suspensions, we report that an instability of a gyrotactically trapped cell layer can arise in a pressure-driven plane channel flow. The linear stability analysis reveals that the equilibrium cell-layer solution is hydrodynamically unstable due to negative microswimmer buoyancy (i.e. a gravitational instability) over a range of biologically relevant parameter values. The critical cell concentration for this instability is found to be 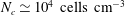 $N_{c}\simeq 10^{4}~\text{cells}~\text{cm}^{-3}$, a value comparable to the typical maximum cell concentration observed in thin layers. This result indicates that the instability may be a potential mechanism for limiting the layer’s maximum cell concentration, especially in regions where turbulence is weak, and motivates the study of its nonlinear evolution, perhaps, in the presence of turbulence.
$N_{c}\simeq 10^{4}~\text{cells}~\text{cm}^{-3}$, a value comparable to the typical maximum cell concentration observed in thin layers. This result indicates that the instability may be a potential mechanism for limiting the layer’s maximum cell concentration, especially in regions where turbulence is weak, and motivates the study of its nonlinear evolution, perhaps, in the presence of turbulence.
JFM classification
- Type
- JFM Rapids
- Information
- Copyright
- © 2019 Cambridge University Press
References
- 5
- Cited by




