Introduction
Pregnancy is a stressful period. Not only do expectant mothers undergo physical changes, they also fear childbirth and are preoccupied about the baby’s health as well as their motherhood Reference Lobel and Dunkel Schetter1 . They may suffer strains in personal relationships Reference Lobel and Dunkel Schetter1 . The worries and stress experienced during pregnancy activate the hypothalamic–pituitary–adrenal axis (HPA), leading to the release of cortisol, a hormone with the ability to cross the placenta and condition fetal development Reference Karlen, Frostell, Theodorsson, Faresjo and Ludvigsson2,Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Delgado-Puertas and Peralta-Ramirez3 .
Cortisol levels have traditionally been evaluated using blood, urine, saliva or amniotic fluid samples Reference Bergman, Sarkar, Glover and O’Connor4–Reference Jung, Ho and Torpy6 . These measurements, however, are punctual and highly affected by the sleep–wake cycle. An alternative is the extraction of hair cortisol: indeed, hair cortisol is the only retrospective biomarker of chronic stress that is unaffected by contextual variables such as noise, temperature or social interaction. It enables retrieving cortisol measurements over the 3 months prior to the date of extraction Reference Jahangard, Mikoteit and Bahiraei7,Reference Wosu, Valdimarsdóttir, Shields, Williams and Williams8 .
During pregnancy, high concentrations of prenatal hair cortisol have been associated with preterm birth, childbirth complications, maternal psychological diseases and wellbeing, postpartum depression and the baby’s neurological development Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Delgado-Puertas and Peralta-Ramirez3,Reference Caparros-Gonzalez, Romero-Gonzalez, Strivens-Vilchez, Gonzalez-Perez, Martinez-Augustin and Peralta-Ramirez9–Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Coca-Arco and Peralta-Ramirez13 . Nevertheless, the relationship between cortisol at the time of conception and the baby’s future sex has been scarcely studied. Studies conducted on the relationship between cortisol and the sexual predisposition of the fetus found high levels of salivary cortisol in the third trimester of pregnancy in women who gave birth to a girl Reference Andersen, Jensen and Schmedes14–Reference Walsh, McCormack and Webster17 . In the same vein, other authors showed that women who had higher cortisol concentrations in their saliva before conception were less likely to have a male baby Reference Chason, McLain and Sundaram18 .
These findings are in fact in line with numerous studies showing the decline of male births in the population following exposures to stressful stimuli such as earthquakes, murders, terrorist acts, stressful work or life-changing events Reference Bruckner, Catalano and Ahern19–Reference Navara27 .
One hypothesis is that parents’ stress modifies the concentration of sex hormones through the activation of the HPA axis and has implications regarding sex allocation. This approach is supported by several experimental studies that have found that the sex of the zygotes is influenced by the stress of both parents around the moment of conception: the higher the stress level, the more likely of giving birth to a girl Reference Chason, McLain and Sundaram18,Reference Navara27–Reference Song, Mohamed and Pang30 .
However, other authors have failed to find such a relationship between cortisol and the baby’s sex Reference Bae, Lynch, Kim, Sundaram, Sapra and Buck Louis31 . The studies linking stress to biological measures and the sex of future babies may be promising, but they are in fact few and far between.
Therefore, further tests are necessary to show whether cortisol concentrations during pregnancy influence the sex of the offspring and whether they do so during conception Reference Navara27 . In fact, to the best of our knowledge, no study, that is not merely punctual (cortisol in saliva, urine or blood), has yet been carried out using a retrospective measure (the mother’s hair cortisol) on the relationship between cortisol and the baby’s sex.
Therefore, the study’s main objective was to check whether a relationship existed between the sex of the offspring and the cortisol secretion in the mother’s hair before and during the baby’s conception, as well as in the first weeks of pregnancy. In a complementary way, a second aim was to know whether psychological stress could be related to sex of the offspring, the levels of psychological and specific stress were evaluated in those weeks of pregnancy.
Methods
Sample size estimation
There are some studies whose aim was to find out the relationship between salivary cortisol and sex of the fetus. Sample size estimation was calculated using this variable in Giesbrecht et al. Reference Giesbrecht, Campbell and Letourneau16 . G * Power Reference Faul, Erdfelder, Buchner and Lang32 was used to calculate the sample size to achieve 80% power and contrast the null hypotheses H n: μ1 = μ2 at the 5% alpha level. Comparing two independent means (t-test) using mean scores and deviation standards of Giesbrecht et al. Reference Giesbrecht, Campbell and Letourneau16 of two groups (male and female), the sample size required were 72 participants, 37 for each group (Cohen’s d = 0.77).
Participants
The total sample was made up of 108 expectant women in weeks 8 and 10 of their pregnancy and there were seven women who were pregnant using a fertility treatment. They were recruited in various health centres of the province of Granada (Spain).
The inclusion criteria were as follow: being an expectant woman in weeks 7 to10 of pregnancy; aged over 18 years and having a minimum of an average level in Spanish. The exclusion criterion was: having a pre-pregnancy illness or taking corticosteroids.
Participation was voluntary, and an informed written consent document was read and signed by every participant. This study followed the guidelines of the Helsinki Declaration (AMM, 2008) and the Good Clinical Practice Directive (Directive 2005/28/EC) of the European Union and was approved by the Human Ethics Research Committee of the University of Granada (reference 881).
Instruments
Sociodemographic and obstetric data were collected from the 2010 Pregnant Woman’s Health Document 33 , which is the official health record for pregnant women and their newborns. The included variables were: age, marital status, educational level, employment status, smoking or not, maternal body mass index, type of pregnancy, number of children, number of previous abortions, whether or not the pregnancy was desired, as well as whether or not it was risky.
Psychological Assessment
Perceived Stress Scale (PSS) Reference Cohen, Kamarck and Mermelstein34,Reference Remor35 . The PSS provides information on the perception of general stress during the preceding month. It consists of 14 items scores on a 5-point Likert scale (0 = never, 1 = almost never, 2 = once in a while, 3 = often, 4 = very often). Spanish reliability alpha’s Cronbach coefficient is 0.81.
Pregnancy Distress Questionnaire (PDQ) Reference Caparros-Gonzalez, Perra and Alderdice36,Reference Yali and Lobel37 : this is a 12-item scale that measures pregnancy-specific stress related to maternal concerns about pregnancy, such as medical problems, labour and delivery, physical symptoms, bodily changes and the baby’s health. Responses are given using a 5-point Likert-type scale where 0 = not at all and 4 = very much. The Cronbach’s alpha reliability coefficient is 0.71.
Stress Vulnerability Inventory (IVE) Reference Beech and Laurence38,Reference Robles-Ortega, Peralta-Ramírez and Navarrete-Navarrete39 . It consists of 22 items that evaluate the person’s predisposition to feel affected by perceived stress. It has a Yes/No answer format. Items receiving an affirmative answer add 1 point. The range of scores on the scale is 0 to 22, higher scores corresponding to greater vulnerability to stress. The scale is highly reliable, with a Cronbach alpha of 0.87.
Chronic stress biomarker: hair cortisol levels
The cortisol evaluation consisted in taking a lock of hair containing approximately 150 strands from the rear corner of the skull, as close as possible to the scalp Reference Sauvé, Koren, Walsh, Tokmakejian and Van Uum40 . A maximum length of 3 cm was set for each sample to reflect cortisol levels during the preceding 3 months Reference Stalder and Kirschbaum41 . The samples were wrapped in aluminium foil to be adequately protected from light and humidity and were kept at room temperature until further analysis using the salivary kit ELISA. The analysis protocol was published in Romero-Gonzalez et al. Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Delgado-Puertas and Peralta-Ramirez3 The lower detection limit was 12.5 pg/mg and the cross-reactivity reported by the manufacturer was as follows: prednisolone 13.6%, corticosterone 7.6%, deoxycorticosterone 7.2%, progesterone 7.2%, cortisone 6.2%, deoxycortisol 5.6%, prednisone 5.6% and dexamethasone 1.6%. No cross-reactions were detected with dehydroepiandrosterone or tetrahydrocortisone.
The intra- and inter-assay precisions were analysed on internal quality controls used for routine salivary cortisol measurement, measured in duplicate in eight consecutive assays. The intra-assay coefficients of variation (CV) were 2.7% at 10.7 ng/ml and 4.3% at 43.9 ng/ml. The inter-assay CVs were 4.4% and 6.3%, respectively Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Delgado-Puertas and Peralta-Ramirez3 .
Procedure
Women were told about the study when they attended their first prenatal appointment with their midwife, 7 to 10 weeks into their pregnancy. Those who agreed to participate were given an information sheet and then signed the informed consent document. Each participant was assigned an identification code in order to ensure anonymity throughout the study. Subsequently, sociodemographic and obstetric information was collected, psychological questionnaires were completed in paper format (PDQ, EEP-1, IVE), and hair samples were then taken applying the established sample collection protocol Reference Sauvé, Koren, Walsh, Tokmakejian and Van Uum40 . Once they gave birth, they were contacted and asked the sex of their baby. This study followed the The Strengthening the Reporting of Observational studies in Epidemiology standards for cohort studies.
Data analysis
The averages and percentages of the most relevant sociodemographic and obstetric variables (age, marital status, educational level, employment status, type of pregnancy, number of children, number of previous abortions and whether the pregnancy was desired, as well as whether it was risky or not) were calculated first.
The participants were then divided into two groups according to the sex of their babies (male or female). Subsequently, in order to check any differences regarding major sociodemographic and obstetric variables between both groups, the t-Student (quantitative variables) and Chi-square (categorical variable) tests were performed.
The data met the assumptions of normality and uniformity of variances (tests of Kolmogorov–Smirnov and Shapiro–Wilk of normality P > 0.05; Levene test to evaluate the homogeneity of variances P > 0.05).
To verify the presence of significant differences in maternal hair cortisol levels between women who had given birth to a girl and those who had given birth to a boy, a comparison was made of independent samples using non parametric Mann–Whitney test. The variable ‘baby’s sex’ was the independent variable, with two levels (female and male). The dependent variables were maternal hair cortisol levels. Besides, to know whether psychological variables were different regarding baby’s sex, a comparison was made of independent samples using the t-Student test. The variable ‘baby’s sex’ was the independent variable, with two levels (female and male) and dependent variables were the scores on perceived stress (PSS), vulnerability to stress (SVS) and pregnancy-specific stress (PDQ).
In addition, calculations were performed, using Cohen’s d, to determine whether the differences between the groups were clinically relevant. We took into account the considerations regarding the interpretation of the effect size magnitudes: d 0.20: size of the small effect; (d) 0.50: medium effect size; d 0.80: large effect size Reference Cohen42 .
The analyses were conducted using the IBM SPSS Statistics for Windows version 25.0. (Armonk, New York).
Results
Sample description
Initially, a total of 178 pregnant women were willing to take part in the study, of which 164 met the inclusion criteria. It was not possible to know the sex of the baby of 21 of them, another 12 decided to leave the study due to a lack of time, and 15 had a miscarriage. In the case of 8 babies (four girls and four boys) of the remaining 116 mothers, the amount of baby hair was deemed insufficient for cortisol analysis, so they were removed from the sample. The final total sample consisted of 108 pregnant women in their first trimester of gestation, aged between 22 and 43 years (M = 33.73; SD = 4.37).
The participants were divided into two groups based on the sex of the baby they gave birth to (male or female). Thus, 46 women (M = 33.41 years of age; SD = 3.83) were included in the baby boy group and a total of 62 women (M = 33.98 years of age; SD = 4.78) were included in the baby girl group.
Table 1 shows the main sociodemographic and obstetric differences depending on the newborn’s sex. No significant differences were found between the two groups in terms of age, marital status, educational level, employment status, type of pregnancy, number of children, number of previous abortions, pregnancy risk or desired/non-desired pregnancy.
Table 1. Differences in sociodemographic variables and obstetric information between pregnant women who had a girl or boy baby
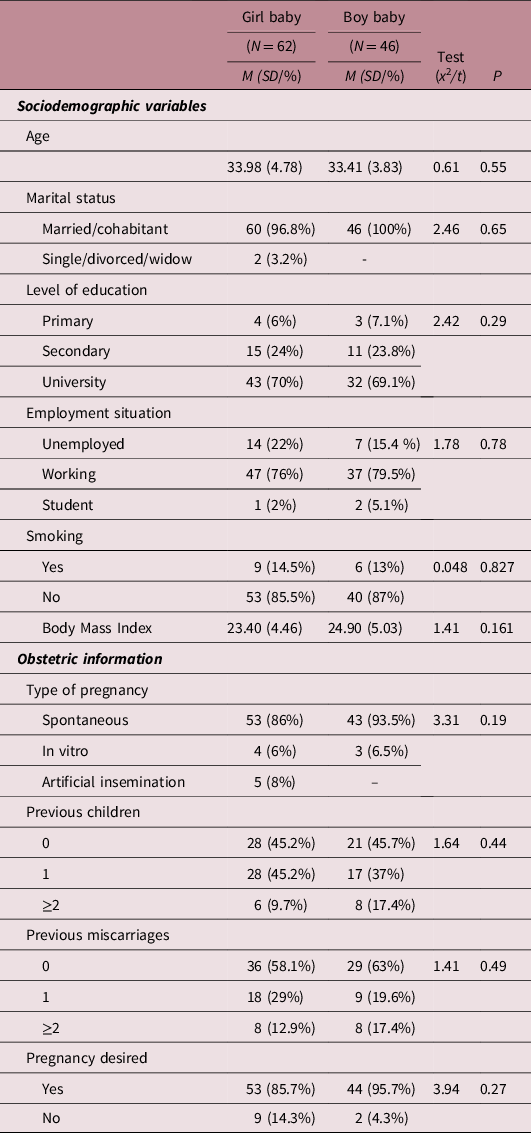
Note: Significance level at P ≤ 0.05. Student t-test used for continuous variables and Chi-squared test for categorical variables.
Relationship between the baby’s sex and hair cortisol levels representing the preconceptional period and the first trimester of pregnancy
Statistically significant differences in maternal cortisol levels were found in the first trimester, depending on the baby’s sex (U = 1097.50; P < 0.05). The levels were higher in the case of baby girls (expressed in median:lower quartile-upper quartile) (164.36:54.45-284.87 pg/mg) compared to that of baby boys (101.13:37.95-193.56 pg/mg). Cohen’s d reported an effect size of 0.40 (Fig. 1).
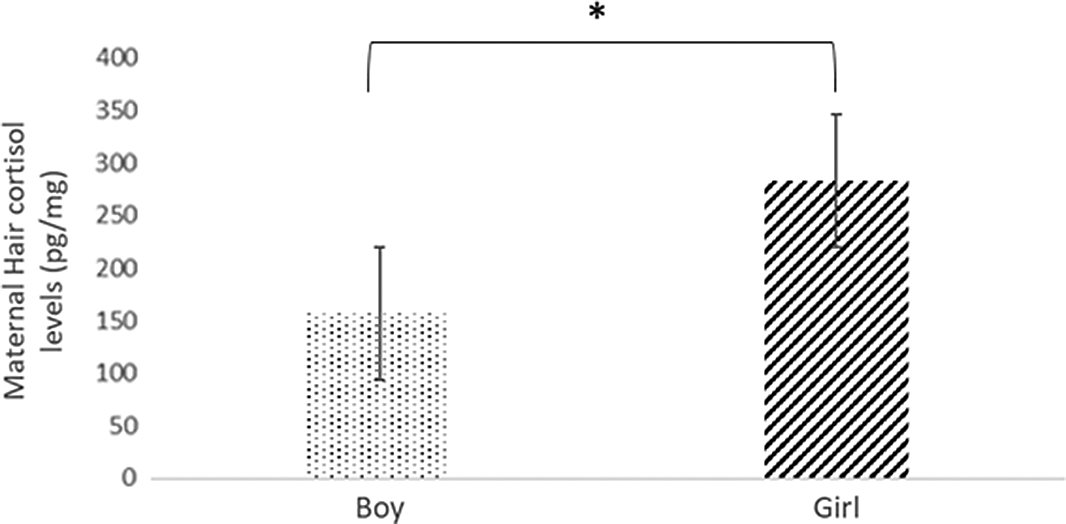
Fig. 1. Hair cortisol levels among pregnant women who had a boy or girl baby.
Note: *significance at P < 0.05.
Relationship between the Baby’s Sex and Stress and Pregnancy Concerns during the First Trimester
There were no significant differences regarding the baby’s sex based on the results obtained in the PDQ (t = 1.46; P > 0.05) or were there any differences in the results of EEP-1 (t = 70; P > 0.05) or in the IVE (t = .08; P > 0.05).
Discussion
The study’s objective was to understand the relationship between the baby’s sex and the level of cortisol in the mother’s hair before conception and in the first weeks of the first trimester, as well as between the baby’s sex and the psychological stress perceived by mothers in the first trimester of gestation.
The results showed differences in maternal hair cortisol levels in the first trimester of pregnancy among women who gave birth to a baby boy compared to those who delivered a baby girl. Specifically, cortisol levels were higher in the first trimester of pregnancy when the fetus was a girl. Therefore, the level of this hormone in the hair in the first trimester of pregnancy seems to be related to the baby’s sex.
When interpreting the results of this study, we must remember that the hormone was extracted from women’s hair between weeks 8 to 10 of their pregnancy. In this way, retrospective information on maternal cortisol levels was obtained during the conception and in the first weeks of pregnancy. Thus, maternal stress was shown to be higher over that period in mothers who later gave birth to a girl as opposed to those who gave birth to a boy.
Previous studies on links between maternal stress during conception and the baby’s sex have produced similar findings: women who presented higher concentrations of cortisol in their saliva prior to conception were less likely to deliver a male baby Reference Chason, McLain and Sundaram18 . Nevertheless, our study seems to contradict other results, such as those of Bosquet Enlow et al. Reference Bosquet Enlow, Sideridis, Bollati, Hoxha, Hacker and Wright43 who have found that maternal hair cortisol levels were higher across the three trimesters when mother have a boy. Future research should focus on this aspect in order to increase knowledge on this topic.
On the other hand, the results showed that there was no difference in perceived psychological stress in the first trimester between mothers who had a boy and those who had a girl. That is, neither the specific stress of pregnancy nor the levels of perceived stress, nor the levels of vulnerability to stress influenced the sex of the baby. These results are in line with that of a previous longitudinal study, in which maternal stress was measured using a series of psychological instruments, and no significant differences were found between the scores of women who gave birth to a boy and those who gave birth to a girl Reference Giesbrecht, Campbell and Letourneau16 .
Thus, the results of the study seem to support the explanatory theories according to which this hormone plays an important role in determining the sex of the baby, both during conception and in pregnancy.
A possible explanation would be that the activation of the HPA axis modifies sex hormone concentrations at the time of conception Reference James and Grech29 . However, the mechanisms underlying this modification are unclear. On the one hand, there is evidence that testosterone functions as a mechanism when determining the baby’s sex, since the greater the prenatal stress levels, the higher the levels of female testosterone Reference Grant28 . Some studies have also focussed on the role of the father’s stress at the time of conception, although we did not take such research into account in the present study. Song et al. Reference Song, Mohamed and Pang30 found that the proportion of sex chromosomes in ejaculated sperm may be altered by exposure to stress, reducing the viability of Y chromosomes, thus affecting the distribution of sexes at birth. Moreover, the X sperm are better at passing through cervical mucus, so when hormonal changes occur, caused by stress, these sperm are greater achievers than the Y sperm Reference Navara27 .
On the other hand, another possible explanation for the results is the theory according to which selective male miscarriages take place during pregnancy Reference James and Grech29 . Our sample, however, did not include pregnant women who subsequently aborted, so we were not able to learn more about this latter relationship.
What does seem clear – and this has been shown in a number of studies – is that fetuses are vulnerable and that stress plays a role. For example, it has been shown that Y fetuses mature more slowly than X fetuses Reference Bale44 ; they tend to present pregnancy complications and preterm birth Reference Walsh, McCormack and Webster17,Reference Rosa, Nentin and Bosquet Enlow45–Reference Zeitlin, Ancel, Larroque and Kaminski47 ; and at birth, they are more likely to have shorter telomeres Reference Bosquet Enlow, Bollati and Sideridis48,Reference Lin, Sun and Wang49 .
The study presented a number of limitations. For example, the role that fathers’ stress may have at the time of conception, which seems relevant in determining the sex of the baby, was not taken into account. Therefore, it would be interesting to include measurements of cortisol in fathers’ hair in subsequent studies. Besides, some authors have detected in older adults that hair cortisol levels are slightly higher in dark brown hair Reference Lanfear, Voegel, Binz and Paul50 , so it could be worth obtaining this information to know if this phenomenon could occur also in pregnant women.
It would also be relevant, in the future, to follow up female participants who were discarded because they aborted. Examining these cases would allow checking whether women expecting boys undergo more stress abortions than women expecting girls, as suggested by recent research. Including this data in future studies would thus lead to a deeper understanding of the mechanisms underlying deviations in the sex of the offspring.
Finally, a future line of research could also be that of reproducing the study with samples of women who present high-risk pregnancies (such as pregnancies resulting from in-vitro fertilisation or artificial insemination). Such populations experience high levels of stress and would allow us to better understand the relationship between stress and the child’s sex.
The findings of the present study suggest that a relationship exists between cortisol levels in mothers at the time of conception and in the first trimester of gestation and the sex of their future babies. Thus, giving birth to a girl would imply higher levels of maternal hair cortisol at the time of conception and in the first weeks of pregnancy. However, no relationship was found between the sex of the offspring and perceived psychological stress levels in the first trimester.
To conclude, the research presented here is pioneering to the extent that it links prenatal stress to the sex of newborns. This was done by measuring pregnant women’s hair cortisol, the only longitudinal measure that is suitable for this purpose. Therefore, this work contributes to an open field of research which requires further studies to explain the role played by prenatal stress and cortisol on sex allocation.
Acknowledgments
Thank you to every pregnant woman who participated in the study. This study is part of a Doctoral Thesis of Mr. Jose A. Puertas-Gonzalez.
Financial support
This work was supported by the Frontier Project “A-CTS-229-UGR18” of the Ministry of Economy, Knowledge, Business and University of the Junta de Andalucía, co-supported by funds/European Regional Development Fund (ERDF) – a way to build Europe. Besides, Mr. Jose Antonio Puertas-Gonzalez has been awarded with an individual research grant (Spanish Ministry of Science, Innovation and Universities, FPU program, reference number 18/00617), as well as Dr. Borja Romero-Gonzalez (Spanish Ministry of Economy, Industry and Competitiveness, FPI Program, reference number BES-2016-077619).
Conflict of interest
None.
Ethical statement
Participation was voluntary, and an informed written consent document was read and signed by every participant. This study followed the guidelines of the Helsinki Declaration (AMM, 2008) and the Good Clinical Practice Directive (Directive 2005/28/EC) of the European Union; and was approved by the Human Ethics Research Committee of the University of Granada (reference 881).






