Introduction
The overarching goal of The Rockefeller Clinical Scholars (KL2) program is to prepare trainees to be successful translational science investigators who improve human health. The program began on an informal basis in 1976, was reorganized into a formal program in 2001, and was modified into the current 3-year New York State-approved Master of Clinical Investigation degree granting program in 2006 at the time of the first Rockefeller Clinical and Translational Science Award (CTSA) and the creation of the Center for Clinical and Translational Science (CCTS). Although a number of outstanding scholars completed the program from 2001 to 2006 and went on to successful academic and research positions at The National Institutes of Health, major academic medical centers, and the Armed Forces Research Institute of Medical Sciences in Thailand, we are limiting this report to the graduates of the Master’s degree program from 2006 to 2016.
Background
The Rockefeller University has been committed to excellence in the conduct and teaching of biomedical research since its founding in 1901 as The Rockefeller Institute for Medical Research. The founding vision was for the faculty to carry out basic research on human diseases, aided by a hospital on the campus devoted solely to research and education. The hospital opened in 1910 with the radically new philosophy of the founding director, Rufus Cole, of staffing the hospital with fulltime, salaried “physician-scientists” performing clinical and translational science at the bedside and the research bench. This model was remarkably successful, and by the time of his retirement in 1937, Cole’s trainees included 112 full-time academic faculty members across the country, 3 deans, 22 members of the National Academy of Sciences, and 46 members of the prestigious Association of American Physicians. The Rockefeller University Hospital carries on this tradition today with virtually all patients in the Hospital participating in a research protocol led by full-time faculty and trainee translational investigators. Patients are not charged for either their medical or hospital services.
Methods
Program Size and Funding
Based on our experience from 2001 to 2006, we found that individual attention to scholars, active participation by scholars in tutorials, peer learning, and social cohesion were optimal when the program contained 15–20 scholars (online Supplementary Fig. S1).
The program has been supported by the KL2 portion of the CTSA and University funds, the latter coming both from a University endowment fund and additional philanthropic support (online Supplementary Fig. S1). Scholars are each paid the same base amount from the program, but mentors can provide modest supplements from their own funds to recognize extraordinary experience, expertise, or responsibilities—or success in obtaining external funding. To insure that the scholars can devote essentially 100% of their effort to their research, they are enjoined from performing clinical services for remuneration (other than overnight and weekend clinical coverage in the Rockefeller University Hospital itself). At the same time, if their clinical research project at Rockefeller is too limited to maintain their clinical skills, they are encouraged to perform limited clinical activities on a voluntary basis at one of the nearby academic medical centers.
Program Philosophy
Translational research differs from basic investigation in that it seeks to use the scientific method to address a health need and so its goal is to improve human health. In contrast, basic science discovery research is designed to critically probe current scientific paradigms with the goal of advancing scientific knowledge. Well-conceived and executed basic science projects always produce new knowledge because they either support or do not support the current scientific paradigms. In contrast, the uncertainties surrounding the translation of a scientific discovery into a novel drug, diagnostic device, or disease prevention strategy, make it unlikely that any single project will be able to accomplish the goal of improving human health. To integrate the strengths of each approach, trainees are encouraged to both pursue their translational hypotheses and collect detailed mechanistic information so that new basic science knowledge will be obtained even if the translational component is not successful in improving human health.
Thus, the guiding philosophy of the Clinical Scholars (KL2) program is that translational investigators require 3 fundamental skills: (1) the ability to articulate a health need with the same precision as a basic science hypothesis. (2) The ability to devise an assay or assays with which to interrogate an important aspect of the scientific basis of one or more phenomena underlying the health need. The assay must be robust and practical, meaning that in addition to having a definitive endpoint, it can be performed reproducibly, in a reasonable time period, and at acceptable cost. In addition, despite its reductionist nature, the assay needs to reflect the essentials of human biology and medical reality so that if some agent or intervention is found to have a positive impact on the assay, it will likely have a similar impact on whole animals, humans, and populations. Since translational research encompasses a wide spectrum, the assay may be at the molecular, cellular, whole animal, human, community, or population level. (3) The ability to conceptualize a pathway from discovery to regulatory approval and/or clinical adoption and implementation. This last component requires a sophisticated understanding of the regulatory process and the ability to rigorously design one or more pivotal studies that meet the highest bioethical standards, are adequately powered statistically, and have a compelling measurable endpoint related to the health need so as to justify regulatory approval and/or clinical adoption and implementation. Thus, the Clinical Scholars (KL2) program is designed to provide an optimal environment for scholars to acquire each of these skills through both experiential and didactic components, coupled with the support of an extensive infrastructure of experts who can guide the scholars through the development, approval, conduct, analysis, and dissemination of the results of their own protocol. Team science is integral to the success of translational research and so it is emphasized throughout the program, most particularly the requirement that all scholars serve as principal investigator (PI) of their own human subjects protocol.
Program Organization
For the years 2006 to 2015 the CTSA PI Dr Barry Coller served as the Clinical Scholars (KL2) program director and Dr Sarah Schlesinger, Associate Professor of Clinical Investigation at Rockefeller, served as codirector. In 2016, the director and codirector changed positions. The director and codirector select a Chief Clinical Scholar (or scholars) each year who helps with organizational and administrative issues, assists with recruitment of new scholars, guides the scholars in their choice of tutorial topics, reviews the scientific quality of protocols as a member of the CCTS Advisory Committee for Clinical and Translational Science, and provides feedback to the program leadership. The chief scholars also play a vital role in leading the comprehensive anonymous review of all aspects of the program by the scholars each year, along with developing and implementing important changes in the program to take advantage of new opportunities and to strengthen elements that need improvement. The Clinical Scholars program administrator, Michelle Romanick, plays a vital role in insuring that the scholars meet the milestones along the timeline required for them to complete the program in 3 years.
Applicant Pool and Selection
The Clinical Scholars (KL2) program is geared primarily for physicians, either M.D.s or M.D./Ph.D.s, who have completed or nearly completed their clinical training, but it is also open to individuals with medically related graduate degrees. For example, scholars have included individuals with Ph.D. degrees in epidemiology, biophysics/optics, and bioinformatics. If mutually agreed to by the Clinical Scholars program director and ACGME-approved residency or fellowship director, scholars can apply their first year in the program toward partial fulfillment of their residency or fellowship research requirements. The application requires a statement of scientific and career goals and a separate detailed letter from a faculty member in the CCTS requesting that the applicant be admitted to the program and providing a description of both the translational project that the applicant will work on and a specific plan for the applicant’s career development. The latter must include a plan agreed to by the applicant and mentor for the period immediately following completion of the program, such as applying for an National Institute of Health (NIH) K08 or K23 award. This insures that the applicant and the mentor have agreed upon a set of career goals from the beginning of the program. The criteria used by the admissions committee, which is composed of the director, codirector, 2 additional faculty members, and the Chief Clinical Scholar(s), in selecting the applicants include their academic performance, their research experience and potential, their letters of reference, their personal statements indicating their career goals in clinical and translational research, and the letter from the proposed mentor describing the research project and the career development plan. Scholars who are accepted hold the title of Instructor in Clinical Investigation at Rockefeller University.
While hypothesis-driven molecular and mechanistic research is a central component of most research projects conducted at Rockefeller, protocols are increasingly driven by the health needs identified by community clinicians and patients and conducted in community-based health care facilities in partnership with them. Trainees are actively encouraged, and taught how, to build these partnerships to maximize the inherent synergism in conducting community-driven and community-based mechanistic studies [Reference Kost1].
To provide flexibility in career choices, scholars without Ph.D. degrees who perform at a high level during the Master’s degree program may apply for entry into The David Rockefeller Graduate Ph.D. Program. If a Scholar is accepted by the Rockefeller graduate school admissions committee, which is separate from the Clinical Scholars program admissions committee, the Clinical Scholars program provides support for an additional 2 years of graduate school training.
Mentors
Rockefeller University is comprised of 82 separate laboratories, each led by a Head of Laboratory who reports directly to the Rockefeller President. Each lab head sets the research program for the lab and is free to change paths as their scientific discoveries lead into different areas without the constraints of traditional departments. Currently, there are 61 Ph.D., 7 M.D., and 13 M.D./Ph.D. lab heads, reflecting the strength of the basic sciences. Basic investigators are invited to conduct human subjects research and supported in developing and conducting their protocols by a strong infrastructure that includes a protocol Navigation program [Reference Brassil2] and extensive resources for conducting the studies in the Rockefeller University Hospital [Reference Kost3–Reference Kost5]. The CCTS also provides access to experts in all aspects of protocol conduct (online Supplementary Table S1).
Of the 21 lab heads who mentored the graduates of the program from 2006 to 2016, 8 hold Ph.D. degrees, 5 held or hold M.D. degrees, and 7 hold both M.D. and Ph.D. degrees. One indicator of the scientific achievements of these mentors is that 10 of them were elected to membership in the National Academy of Sciences, 3 received Lasker awards, and 1 was a Nobel laureate. In addition, 5 of the 21 mentors have been supported by the Howard Hughes Medical Institute and the 19 mentors who are currently active at Rockefeller have a total of more than $250 million in NIH grant support. Thus, the laboratories that the Clinical Scholars join are well supported and are led by distinguished scientists who have been successful in obtaining peer-reviewed support.
Core Elements of the Program
The core elements of the Clinical Scholars (KL2) program are listed in Table 1 and some of the individual elements are discussed in the online Supplementary Materials, including Mentored Clinical and Translational Protocol; Tutorial in Clinical and Translational Science; Seminars in Clinical Research and Luncheon Meetings with Speakers; Biostatistics/Bioinformatics, Epidemiology, and Research Design; Pilot Project Grant Writing; Manuscript and Grant Writing Workshops; Graduate School Course; Team Science Training; Humanities and Translational Science; Evaluation by Master’s Degree Advisory and Review Committee (MARC); and Career Development and Individual Development Plans.
Table 1 Core elements of Clinical Scholars (KL2) program

Special Features of the Program
In recognition of the importance of trainees being able to devote 100% of their effort to advancing their research careers, Rockefeller University provides all Clinical Scholars access to University-owned nearby housing at competitive rates and an on-campus daycare facility at rates geared to family income. In addition, since medical school debt may have a negative impact on trainees’ career choices, the program has recently initiated a loan forgiveness program for graduates to supplement the NIH program. This program provides a single payment equal to 50% of the Scholar’s remaining medical school debt at the time of graduation from the program, up to a maximum of $60,000, contingent on the Scholar also applying for the NIH program. To assist foreign trainees who want to continue their training or careers in the United States, the program also provides partial support for the waivers and applications required to become permanent residents.
Tracking Graduates of the Program
Soon after initiating the Clinical Scholars (KL2) program we realized that there was little standardization of the graduate tracking process, with no generally recognized tracking tool available and each institution developing its own system. We realized that it would likely be difficult to achieve high response rates to periodic surveys from graduates because of the time and effort required to complete the surveys amid competing time demands. To address these limitations, with feedback from CTSA members at other Hubs, we created a Graduate Tracking Survey System (GTSS) as a web-based, “smart” computerized survey to track the career progress of Clinical Scholars after they graduate from the program [Reference Romanick6]. The philosophical basis for the selection of the questions is our belief that the essential criterion by which to judge the success of a translational science training program is whether graduating trainees go on to improve human health. As a result, many questions are designed to assess this directly. However, since there is expected to be a significant time lag between when a trainee completes the program and when she or he improves human health, other questions are designed to assess “surrogate” indicators that may provide valuable interim measures of likely success. The GTSS pre-populates data on grants and publications in standardized formats to reduce the time required to complete the survey. It was designed to be readily adapted for use at other institutions and at present, 25 other CTSAs have adopted the survey.
Results
Data on Graduates of the Clinical Scholars (KL2) Program From 2006 To 2016
Data on the 40 graduates of the Clinical Scholars (KL2) program from 2006 to 2016 are provided in Table 2, online Supplementary Table S2, and Fig. 1.

Fig. 1 Clinical Scholar graduates. (a) Positions of 2006–2016 Clinical Scholars program graduates at the time of graduation. (b) Current positions of 2006–2016 Clinical Scholars program graduates. (c) Cumulative publications of Clinical Scholars to 2016. Data depicted as box plots with the median value indicated by the line in the box; the mean indicated by the + sign in the box; the box encompassing 50% of the values; and the extensions encompassing the upper and lower values.
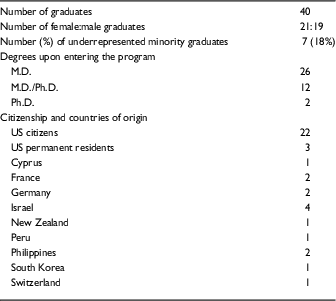
Table 2 Demographic data on Clinical Scholar (KL2) graduates 2006–2016
Demographics
There was nearly equal sex distribution with 21 female and 19 male graduates. In total, 26 Clinical Scholars had M.D. degrees on entering the program, whereas 12 held M.D./Ph.D. degrees, and 2 held Ph.D. degrees. Seven of the 40 scholars (18%) were from underrepresented minority groups. Out of the 40 graduates, 22 (55%) were US citizens and 3 (8%) were US permanent residents; the remaining 15 (38%) came to the United States from 9 different countries, with more than one coming from Israel (4), Germany (2), France (2), and the Philippines (2). Five of the 40 scholars (13%) used their first year of participation in the program in partial fulfillment of the close-up research requirement for their US clinical residency or fellowship. The areas of focus of the scholars are shown in the online Supplementary Table S2. There was a wide distribution of disciplines; the areas most heavily represented were infectious diseases (6), dermatology (4), rheumatology (4), endocrinology (3), and gastroenterology (3).
The positions Clinical Scholars held at the time of graduation from the program and at the time of writing are shown in Figs. 1a and b. Out of 40, 18 (45%) of the scholars remained at Rockefeller immediately after graduating, whereas 9/40 (23%) took faculty positions in the United States or Canada (6) or in their native country (3). Out of 40, 3 (8%) US citizen graduates returned to, or started US ACGME-approved training programs, 1/40 (3%) US graduates entered a postdoctoral fellowship at a US academic institution to obtain additional training, and 2/40 (5%) non-US graduates decided to repeat their clinical training in an ACGME-approved US clinical training program so that they could obtain US licensure. In the latter case, all of the trainees planned translational careers, including basic mechanistic studies. Out of 40, 4 (10%) graduates entered industry, 2/40 (5%) entered government service, joining the US Agency for International Development and the San Francisco Department of Health, and 1 went into the private practice of gastroenterology.
The positions of the Clinical Scholars at the time of writing (Fig. 1b ) shows a shift to a higher percentage (42%) of trainees in faculty positions in US and Canadian academic medical centers, including the ones at Harvard Medical School, Icahn School of Medicine at Mount Sinai, Memorial Sloan Kettering Cancer Center, New York University School of Medicine, Northwestern University, Stanford School of Medicine, the University of Pennsylvania, the University of Pittsburgh, and Weill Cornell Medical College (online Supplementary Table S3). Out of 40, 2 (5%) additional scholars chose research positions in industry, yielding 6/40 (15%). Those industry translational research positions are at Bristol-Myers Squibb, Genentech, Amgen, Celgene, CLINiLABS, and Neurogenetics. Their titles include Clinical Research Director; Medical Director; Director, Exploratory Translational Research; Executive Director of Clinical Research and Development; Associate Medical Director; and Director of Medical Sciences.
The 4/40 (10%) of scholars who returned to their native country after participating in the Clinical Scholars program have all competed successfully for academic positions and are continuing to participate in clinical and translational science. They hold positions at Hadassah Hospital, Hebrew University, Israel; University of Cologne, Germany; Beilinson Hospital, Rabin Medical Center, Israel; and Institute of Molecular Biology and Biotechnology, University of the Philippines National Institutes of Health. Their titles include Head, Department of Medicine; Associate Professor of Medicine; and Associate Professor of Experimental Immunology.
Overall, scholars showed good progress in their records of publication, but with considerable variability in the cumulative number of publications as of 2016 (median 14; Fig. 1c ). In general, the 15/40 (38%) scholars who came to the United States after attending medical school and obtaining some clinical training abroad had stronger publication records before entering the program and they maintained their publication productivity after graduation (median 26 vs. 12, p<0.002; Wilcoxin). As expected, the 11/40 (28%) scholars who entered the program with M.D./Ph.D. degrees had stronger publication records on entry than those with single M.D. or Ph.D. degrees and so their cumulative number of publications trended higher (median 19 vs. 12; p=0.10). There are, however, limitations to the publication record as an index of contributions to translational science. For example, the 6/40 (15%) graduates who joined industry after graduating trended to having fewer publications (median 11 vs. 15; p=0.42), but they lead the development of exciting new medications.
Graduates have been lead authors on major publications in the most prestigious journals and are leading paradigm changing research programs (see Clinical Scholar Graduates Publications and Scientific Programs in online Supplementary Materials).
Six scholars admitted to the program between 2006 and 2016 did not graduate from the program. The reasons for leaving the program varied widely. One Scholar received an outstanding offer to lead a program in genomic and molecular pathology and has continued on in a translational research career. A second Scholar returned to an academic position because of the untimely death of his Rockefeller mentor and has continued on in a successful academic career. A third Scholar with an entrepreneurial orientation received an attractive offer from industry and has continued in a translational research career in industry. A fourth Scholar, who came from another country and had a distinguished prior research record, decided that it was vital to his career goals as a physician scientist to obtain a US license to practice medicine and so he entered an internal medicine program at a major US academic medical center. One Scholar became a clinical gastroenterologist and is now on the teaching faculty of a medical school. The last Scholar decided that he did not want to pursue a research career and so he entered the private practice of dermatology.
Table 3 summarizes the success of the graduates who are in academic positions in the United States in obtaining NIH peer-reviewed funding, including the type of award and the distribution by Scholar degree and sex. To date, they have obtained 23 grants and awards for a total of $23 million. These include 6 different K series grants, with K08 (6) and K23 (4) most common. Graduates have also obtained 8 R and U series awards. Given the national concerns about the competitiveness of women in science [Reference Ley and Hamilton7, Reference Jagsi8], we are gratified that the female graduates of the program have been awarded 9 of the 14 K awards, all 4 of the R awards, and 2 of the U awards. Similarly, given the concern about the competitiveness of M.D. investigators [Reference Schafer9], we are gratified that M.D.s represented 11 of the 14 K awards, 2 of the R awards, and 2 of the U awards.
Table 3 NIH grant support for Clinical Scholar graduates 2006–2016

Scholars in the United States have also been successful in obtaining more than $20.3 million peer-reviewed grant and career development award funding from major US foundations, professional societies, and related organization. These include the Gates Foundation, the Chan Zuckerberg Initiative, the American Association for Cancer Research, the Crohn’s and Colitis Foundation of America, the Patient Centered Outcomes Research Institute (PCORI), the National Organization of Rare Diseases, the Doris Duke Foundation, the Dana Foundation, and the Simons Foundation for Autism Research. Thus, the total peer-reviewed funding for the 25/40 Scholar graduates in academic positions in the United States is $40.8 million.
The 4 scholars who returned to their native countries have also been successful in competing for government and foundation awards, including the ones from the European Union and organizations in France, Germany, and Israel totaling more than $6 million.
Discussion
In this report we summarize more than a decade of experience with the Clinical Scholars (KL2) program at Rockefeller University. Our program reflects our attempt to maximize the strengths of our institution, which include small scale, outstanding faculty and resources, and strong financial support from the institution, in service to our educational mission. Thus, we emphasize experiential learning and close Scholar-mentor relationships. We have also built a strong infrastructure to support the development, conduct, and analysis of research protocols and use it as a key component in educating scholars, especially with regard to team science and team leadership. In addition, we have reached out to the basic science lab heads at our institution to encourage them to participate as mentors. In the latter circumstances, the director and codirector take on the responsibility of co-mentoring scholars on the medical aspects of career development.
Rockefeller University differs from academic medical centers in not having a medical school or residency or fellowship training programs. As a result, we do not have a pool of graduates from such programs as potential Scholar applicants. Nor do we have a large number of departments recruiting junior faculty with demonstrated research records on an ongoing basis. In fact, none of the scholars held an NIH grant at the time they entered the program. On the other hand, our University funding makes it possible for us to support trainees from other countries, many of whom have strong research records, and this strengthens the diversity of our program, with tutorials often incorporating discussions about cultural, academic, and medical differences among the diverse group of countries represented around the table. Another byproduct of our lack of a ready internal pool of applicants is the predominance of trainees who are MDs with only limited research experience. These “late bloomers,” who have made a commitment to a translational research training career after completing their clinical training, are extremely enthusiastic, but their success is less assured. We are very gratified, therefore, that the M.D. graduates of our program have been so successful in obtaining research positions and peer-reviewed research funding. We are also pleased that 42/45 (93%) of the scholars admitted to the program and 39/40 (98%) of the graduates of the program are still actively engaged in conducting translational research.
Since the CTSA KL2 program is still in its early stage, it is especially important that the progress of graduates is carefully tracked. The GTSS we have developed and shared with 25 other institutions is a very valuable tool in obtaining updated information. While each training program is unique, it may be valuable to have comparable data from other sites for the purposes of benchmarking and identifying best practices. Because the GTSS obtains data on grants, publications, and clinical research studies in standardized formats, it has the potential to aggregate data from multiple sites if in the future individual programs agree to share their data.
The major limitation of our review is the lack of a comparable control group of aspiring translational investigators to ascertain the impact of our training program. While it is theoretically possible to perform such an experiment, for example by randomly selecting one-half of a pool of top candidates twice the size of the incoming class, such an experiment raises major issues of fairness and equity. One common criterion of success is based on the ability of graduates to obtain external peer-reviewed support. Of the 26/40 scholars who are in academic positions, 20 have received either peer-reviewed foundation or governmental support. Among the 35 graduates who were eligible to apply for NIH K awards at the time of graduation, 2 entered government service, 2 joined industry, 5 returned to or started a clinical training program, 1 chose to apply instead for an R21 (and was successful), and 1 went into private practice. Of the remaining 24 graduates, 18 (75%) applied for a K award, and 13 of the 18 (72%) were successful. It is still quite early to judge the success of our graduates in obtaining grant funding, especially as regards those who graduated most recently.
Our graduates have obtained 4 R01 grants as PI or Co-PI and 1 R01 as a Co-Investigator. Since the average age of M.D. investigators at the time of their first R01 grant in 2016 was ~45 years of age whereas the current average age of our Scholar graduates is 42.5 (±4.3) years, and since many of our trainees join us immediately after or during fellowship with relatively little research experience, it is too early to judge their ultimate success in obtaining an R01. This is highlighted by the example of one of the graduates who took an academic position immediately upon graduating in 2009 and did not apply for a K award, but 7 years after graduation received an R01 grant.
Among those who remained at Rockefeller after graduating, either fulltime or by retaining an affiliation with the laboratory in which they trained, it is heartening that several have taken advantage of the intellectual freedom that is one of the fundamental principles of the institution. Thus, one nephrologist trainee with an interest in atherosclerosis in patients with end-stage renal disease discovered an important molecule involved in immune cell trafficking and then successfully pursued the potential of this agent in an animal model of multiple sclerosis. Similarly, a gastroenterology trainee developed a metagenomics approach to identifying novel products from intestinal bacteria and found an interesting molecule that has a major impact on glucose metabolism. The ease with which these trainees transitioned between what are ordinarily considered separate disciplines is impressive and will serve them well as they try to maximize the health-related benefits of their discoveries. To assist the graduates of our program who chose to remain at Rockefeller achieve full scientific independence, we established the Rockefeller Early Phase Physician Scientist (REPPS) program to complement the Clinical Scholars (KL2) program. This program is led by the REPPS members and focuses on topics in career development, with an emphasis on grant writing. REPPS members also serve as especially valued mentors for the Clinical Scholars.
The training of translational investigators, and in particular, physician scientists, is one of the highest priorities of the US biomedical research enterprise because these individuals play a vital role in synthesizing both basic science and clinical knowledge in the pursuit of improving diagnosis, treatment, and prevention of disease [10]. Many factors have made it increasingly challenging for individuals to acquire the requisite knowledge and skills, and to compete successfully for academic positions and peer-reviewed grant support [Reference Schafer9]. Our approach has been to focus on experiential training with one-to-one senior mentorship, complementary tutorials focused on specific skills required for success, and a robust translational research infrastructure composed of individuals committed to physician-scientist training to support and guide trainees through the design, execution, analysis, and dissemination of the results of their human subjects protocol. Thus, trainees learn team science by serving as team members and team leaders as their studies evolve. Moreover, we specifically identify our program as a career development program rather than as a postdoctoral research experience and reinforce the importance of strategic career planning even before the trainee is admitted into the program. We recognize that Rockefeller University differs from academic medical centers in scale and focus, but we believe that many of the core principles of our program are broadly applicable, with individualization for institutional culture, priorities, and resources.
The KL2 program initiated by the NIH under the CTSA program was specifically designed to train translational investigators. While it is still very early to assess the success of this initiative in improving human health, we believe the positive surrogate indicators from the graduates of our program from 2006 to 2016 auger well for the long-term impact of this program.
Acknowledgments
The authors thank Suzanne Rivera for outstanding administrative support.
Financial Support
The Clinical Scholars program received funding from The Rockefeller University Center for Clinical and Translational Science (RUCCTS) sponsored by the National Institute of Health, National Center for Advancing Translational Sciences grant UL1TR001866, KL2TR000151, The Rockefeller University Clinical Scholars Endowment Fund, The Dracopoulos Science and Society Initiative at The Rockefeller University, The Iris and Jumming Le Foundation, The Vilcek Foundation, The Leona M. and Harry B. Helmsley Charitable Trust, The Sackler Center for Biomedicine and Nutrition Research through the generosity of the Sackler Foundation, The Shapiro-Silverberg Fund for the Advancement of Translational Research, The Bernard L. Schwartz Program for Physician Scientists, and The Hirschl Trust.
Disclosures
The authors have no conflicts of interest to declare.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cts.2017.308








