Introduction
Research conducted in academic institutions holds great promise for the development of new innovations in therapeutics, medical devices, in vitro diagnostics, and health care information technology. However, getting an idea out of a research laboratory and into a viable commercialized product is difficult and requires expertise that most academically trained scientists do not have. In order to address this situation, the National Institutes of Health National Center Advancing Translational Sciences created the Clinical and Translational Science Awards (CTSA) to support institutions in the development and maintenance of “integrated intellectual and physical resources for the conduct of original clinical and translational science [1].” The Institute of Translational Health Sciences (ITHS), formed as a partnership between the University of Washington (UW), Fred Hutchinson Cancer Research Institute (“Fred Hutch”), and Seattle Children’s Research Institute (SCRI), is one of the largest CTSA-funded programs [2], spanning a geographical area that includes Washington, Wyoming, Alaska, Montana, and Idaho (WWAMI). The ITHS offers its resources to researchers in the WWAMI institutions linked to the Regional Medical School Program at the UW.
The Drug and Device Advisory Committee (DDAC) is one of the mentoring programs created by the ITHS to help train biomedical researchers in the translational sciences [3]. The DDAC consults with individual investigators at multiple time points, serving as a “virtual” team member to advise investigators on the scientific and regulatory requirements for product development. The DDAC focuses on the preclinical stages of development, with emphasis on refining the clinical indication and the scientific and regulatory requirements related to product development, such as manufacturing, safety testing, animal models, analytic methods, pharmacokinetics, and biomarker assay development and validation. The specific aims of the DDAC are to:
-
1. Create and maintain a collaborative network of experts and external resources that collectively enable and support translational research within the ITHS member institutions and broader research community; and
-
2. Develop a robust consulting mechanism that provides expert scientific and regulatory guidance to investigators seeking to translate their research innovations into viable medical products.
In addition to the DDAC mentoring program, the ITHS partnered with the UW Regional Primate Center and the UW School of Pharmacy to co-fund two Ignition Awards supporting preclinical translational research. DDAC members participate in the review of applications and consult with the teams selected for funding. A third initiative, developed in partnership with UW CoMotion, the UW Buerk Center for Entrepreneurship and the Washington Research Foundation (WRF), is the ITHS & WRF Commercialization Summer Fellowship program (“Fellowship”), which provides a mechanism for investigators to obtain detailed commercialization plans for their projects. The DDAC provides regulatory guidance to the selected teams. This multipronged approach is intended to provide investigators with individualized information and resources needed to navigate the complicated path toward development of their products.
In 2013, the Institute of Medicine reviewed the CTSA program and recommended some refinements, including additional emphasis on models of team science and entrepreneurship [4]. The Institute of Medicine also called out the importance of disseminating innovative research models to other institutions involved in similar efforts. In order to evaluate the relative impact of its programs, the DDAC undertook a retrospective analysis of the progress made by each of the product teams it supported between 2008 and 2016. Tracked metrics included the number of investigators who consulted with the DDAC, met with the DDAC more than once (“return metric”), initiated a clinical trial, and commercialized their project by licensing their intellectual property (IP) and incorporating a new company (“startup”) and/or were successful in obtaining follow-on funding from internal pilot awards, federal small business innovation research grants or venture capital. This review also compares the commercialization data from the UW School of Medicine and the Fred Hutch.
Methods
DDAC
The DDAC meets twice a month, with occasional ad hoc meetings to support investigators with time-critical requests. DDAC meetings include the technology manager from the institution’s technology transfer office to ensure that any relevant information about the project’s intellectual property and commercialization prospects is shared. DDAC consults are available to investigators in any of the ITHS partner institutions. There is no charge for the consultations, and investigators are invited to return as their projects advance. Teleconferencing is offered to investigators outside of the Seattle area. All DDAC members are under confidentiality agreements with the UW for their participation on the committee. Meetings are scheduled for 1.5 hours so there is ample time for discussion. The Director of Research Partnerships (“Director”) guides the conversation so that it stays focused, is solution-oriented, and remains a “safe place” for the investigator.
DDAC Member Qualifications
The DDAC is composed of 11 individuals with extensive prior experience in the biotech, pharma, or medical device industries. Some members are permanent and others are invited based on the specific needs of a project (e.g., medicinal chemistry). The Seattle area is fortunate to be home to many companies in the medical device and therapeutic industry, which serve as the source of many of its members. DDAC members also have multiple contacts within the regional biotechnology and pharmaceutical industry to help facilitate relationships between ITHS investigators and potential corporate partners. As currently funded, permanent DDAC members receive 4%–5% salary support to participate on the DDAC. External consultant members are provided with a consulting fee of $150 per meeting. Some members donate their time to the committee. The expertise represented on the DDAC covers many components of preclinical research and development, including biomedical regulatory affairs, formulation and pharmacology, toxicology and pharmacokinetics, medical device development and regulatory affairs, medicinal chemistry, intellectual property, internal medicine and safety monitoring. DDAC members may contribute to the review of local gap funding initiatives or to other ITHS programs, particularly if there are questions related to the regulatory requirements for product development.
Director of Research Partnerships
The DDAC Director of Research Partnerships (“Director”) plays a key role in bringing projects to the DDAC. The Director meets regularly with representatives from the technology transfer offices of participating institutions and participates in the review of many local gap funding initiatives. The Director also attends a variety of university-based and regional commercialization events as a representative of the ITHS and spokesperson for the DDAC consulting program.
Prior to scheduling a DDAC meeting, the Director meets with the investigator and their technology manager to review the status of the project. An investigator whose research is in the discovery phase may request a DDAC meeting, but in some cases it is more expedient for the Director to arrange a one-on-one with a DDAC member to discuss a specific question. The Director is responsible for documenting the recommendations of the committee. The Director also tracks all requests for consultations with the full DDAC or individual members. The role of Research Partnerships Director is a full-time position.
Ignition Award
The ITHS partnered with the UW Regional Primate Research Center and the UW School of Pharmacy to create two pilot awards supporting preclinical research. The Primate Ignition Awards are intended to generate preliminary data to serve as a basis for submission of new research grant applications and/or subsequent clinical studies. Three awards of up to $75,000 are awarded annually. Awardees of the Pharmacy Ignition Award were partnered with a School of Pharmacy faculty member to advance their translational research in areas of drug delivery, transport, metabolism, pharmacokinetics, or pharmacogenetics. Two awards of $40,000 each were awarded annually for a total of five years. These awards were co-funded by the School of Pharmacy and ITHS. All Ignition Award applications were reviewed and selected by a team consisting of DDAC members and representatives from the partner organizations. The Pharmacy Ignition Award is no longer active due to loss of funding.
ITHS and WRF Commercialization Summer Fellowship Program
The goal of the ITHS and WRF Commercialization Summer Fellowships [5] is to create a package of work and information that follows the technology through its commercialization path. This program is co-managed with WRF, CoMotion, DDAC, and the UW Buerk Center for Entrepreneurship. The ITHS only funds fellowships for health-related technologies, while WRF has a wider mandate. Typically, 6–8 technologies are selected each year for consideration, although only 5–6 fellowships are awarded, depending on available funding and interest by and for the applicants. Selected teams are actively considering commercialization of their product and the inventors must be available to work with the fellow. In this way, the inventors learn from the fellows and the fellows understand more of the technology focus from the inventor. Descriptions of these technologies are posted as part of the application process to gauge interest of the applicant. Applicants are asked to prioritize the technologies by personal interest and submit a cover-letter and résumé explaining their qualifications and interest in a particular technology.
Selected fellows are supervised by advisors from the DDAC and WRF, the Buerk Center for Entrepreneurship, and CoMotion, along with the technology inventors. There is a defined schedule and work plan that students work through for each technology over the summer. The work plan includes modules in technology discovery and evaluation, market opportunities, product and regulatory assessment, and business and revenue modeling. Fellows work on individual projects but share working space and resources as a cohort, allowing for collaborative learning from each other. Fellows also present their findings to each other and program mentors, including DDAC members, upon the completion of each module in the work plan. Fellows attend regular update meetings with the investigative team and advisory teams. At least two follow-up feedback meetings are scheduled with the Buerk Center for Entrepreneurship to share information and resources, and to deliver a final presentation at the end of the program. The expectation is that the Fellowship positions require a minimum of 30 hours a week, over the summer quarter. The fellowship currently pays $10,000 total per student for the summer.
Results
DDAC Consultations
From 2008 through 2016, the DDAC met with 116 individual project teams. The number of total meetings, including premeetings and follow-on meetings was well over 3 times that number. Consultations with the DDAC have steadily increased from a low of 7 new teams in 2009 to 26 new teams in 2016 (see Fig. 1).
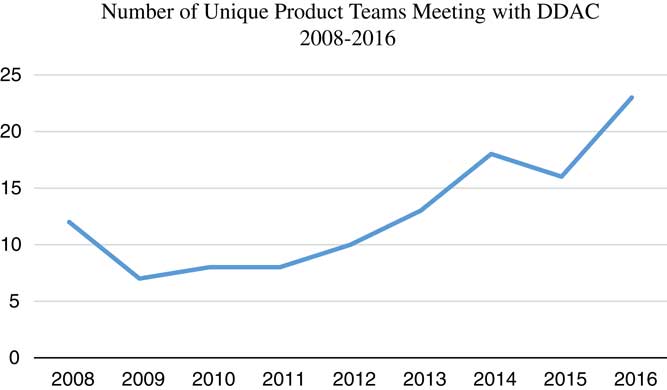
Fig. 1 Numbers of unique product development teams meeting with the Drug and Device Advisory Committee (DDAC) per year. This graphic does not include follow-on meetings with those teams or introductory meetings with the Director of Research Partnerships or Institute of Translational Health Sciences Navigator.
In the first years of DDAC operations, the number of new teams requesting consultations was lower than expected (Fig. 1) given the large number of participating institutions and the fact that the UW is one of the highest funded biomedical research institutions in the country [6]. When investigators were questioned about their current or planned use of ITHS resources, 3 factors emerged that were directly relevant to utilization of the DDAC, listed here in descending order of frequency:
-
1. many investigators did not know that the ITHS or DDAC programs existed;
-
2. many investigators and technology managers felt it was simply “too early” to engage in discussions about product development, that their projects were not “ready,” and
-
3. investigators affiliated with the ITHS, but located at institutions outside of Washington (e.g., WAMI) voiced concerns about maintaining the confidentiality of their unpublished data by sharing it with the DDAC.
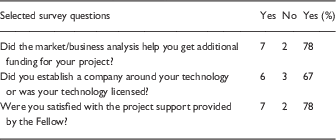
In 2014, a subset of investigators who had met with the DDAC between the years 2012 and 2014 were interviewed about their experiences. Responses to the questions in the survey are summarized in Table 1. These investigators and their technology managers were also asked to provide feedback on DDAC operations.
Table 1 Quantitative analysis of Drug and Device Advisory Committee (DDAC) interviews (n=17 investigators)

Investigators offered the following suggestions for improvement of the DDAC experience:
-
∙ addition of new members to the DDAC with expertise in reimbursement, and health care information technology;
-
∙ provide more team support, in the form of project managers;
-
∙ facilitate access to good laboratory practice (GLP) facilities for the conduct of preclinical toxicology; and
-
∙ offer more investigational new drug (IND)/investigational device exemption (IDE) regulatory writing support.
Input from the CoMotion technology managers included:
-
∙ integrate the DDAC Director of Research Partnerships into New Ventures meetings with CoMotion technology managers to more quickly identify UW investigators that are advancing their translational programs with the goal of forming a start-up company; and
-
∙ develop written action items following DDAC meetings for the investigator and technology manager.
Clinical Development
Of the 116 teams that met with the DDAC to discuss their programs, 47 were conducting research on potential therapeutics (41%) and 33 (29%) on new medical devices. This tally of medical devices does not include in vitro diagnostics (n=12) or mobile phone applications (n=3). Of the therapeutic projects, 10 involved repurposing of existing therapeutics or cGMP production of existing research or veterinary grade compounds. The repurposing teams came to the DDAC with questions related to manufacturing strategies, clinical study design, and IND submissions. The novel therapeutic teams came with a much broader set of questions related to preclinical development, particularly in areas of pilot safety and pharmacokinetics. Out of all of the therapeutic teams only 4 had initiated clinical studies within the timeframe of this review, and 3 of those were studying repurposed compounds. Within the group of repurposed compounds, the primary reason for not pursuing clinical studies was lack of efficacy in animal models of disease. Only 1 of the novel therapeutics supported by the DDAC is currently in clinical trials following incorporation and significant investment from venture capital sources. Out of the 47 teams developing therapeutics, 16 incorporated with the intent of commercializing their IP.
Among the 33 groups developing medical devices, 4 had initiated clinical studies within the timeframe of this review and 15 had incorporated with the intent of commercializing their IP. All but 1 of the device studies were conducted after incorporation and after the teams had obtained sufficient funds to proceed with the trials.
Looking specifically at teams that did not meet with the DDAC, but were recipients of an Ignition Award (n=24) or Fellowship (n=21), an additional 7 startups were incorporated, 4 developing therapeutics, 2 health-related software applications, and 1 medical device. None of these companies had initiated clinical studies by the end of 2016.
ITHS and WRF Commercialization Summer Fellowship Program
A total of 53 fellowships have been awarded since 2008, 35 funded by ITHS, the remainder by the WRF (n=15), Seattle Children’s Research Institute (n=2), or CoMotion (n=1). As part of an investigator satisfaction survey conducted in 2014, 9 out of the 14 investigators paired with a commercialization Fellow were interviewed. The information obtained from the investigators was supplemented with an interview with the Director of the UW Buerk Center for Entrepreneurship. The data are assembled in Table 2.
Table 2 Quantitative analysis of interview responses for commercialization summer fellowships (n=9)
Based on the comments from the investigators and business school several modifications were instituted for the Fellowship program. These included:
-
∙ inviting PIs to participate in the selection of Fellow, if available;
-
∙ developing an “open house” to explain the process and involvement of the Buerk Center and ITHS;
-
∙ development of a more structured set of deliverables and more access to professional advisors;
-
∙ increase in the amount of the student stipend to make it more competitive with other summer fellowship programs.
Return Metric
The data from the investigator interviews indicated that 82% sought follow-on advice from 1 or more DDAC members. This was a surprisingly high number and highlighted a deficiency in the tracking system in place at the time. Since the number of investigators interviewed was small compared with the total number of teams, the return metric was collected for all teams that met with the DDAC between 2008 and 2016. To date, a total of 80 (70%) individual teams have requested follow-on meetings with the full committee or selected members of the committee. The number of follow-on consults ranged from 1 to 5 meetings per team and were spaced ~6–12 months apart. The subset of teams that formed a startup after meeting with the DDAC (n=30 out of 46) accounted for the majority of “repeat” requests, with 87% of those teams requesting follow-on meetings. Of the remaining companies that met with the DDAC (n=16), 9 were incorporated at the time of their first meeting with the DDAC. Three of those teams requested follow-on meetings. The remaining 7 companies were developing products outside the main expertise of the DDAC members (e.g., health care information technology platforms and other data collection tools) and no follow-on meeting was warranted.
Commercialization Data
Of the 161 teams that participated in at least one of the DDAC-supported programs (e.g., DDAC consult, Fellowship, or Ignition Award), 46 (28%) licensed their technology with the intent of commercializing their medical product. The majority of those companies came out of the UW (n=37) or the Fred Hutch (n=3). The DDAC supported half of all UW startups (37/71) and 25% of all Fred Hutch startups (3/12). Other ITHS-affiliated institutions that commercialized a medical invention and sought DDAC support included Seattle Children’s Research Institute (n=2), Washington State University (n=1), and the University of Montana (n=1).
The percentage of UW startups supported by the DDAC increased from a low of 14% in 2011 to 100% in 2016. This increase is attributed to the integration of the DDAC into the UW CoMotion New Ventures program in 2015. The New Ventures program focuses marketing and financial support on projects that are thought to have a high chance of being commercialized but are unlikely to do so without a combination of gap funding, marketing, preclinical, or regulatory support [7]. The partnership between the DDAC and New Ventures ensured that all projects selected for the support were offered the opportunity to meet with the DDAC and discuss the preclinical and regulatory aspects of their programs. Over half of these teams were subsequently successful in obtaining external funding that enabled them to advance their commercialization plans (Table 3). The overall breakdown of support is provided in Table 3.
Table 3 Support of entrepreneurial medical product teams 2008–2016 by Institute of Translational Health Sciences (ITHS) programs
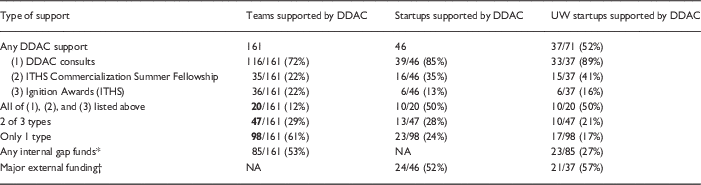
DDAC, Drug and Device Advisory Committee; ITHS, Institute of Translational Health Sciences; UW, University of Washington.
* Includes Ignition Award, CoMotion Commercialization and Innovation Awards, and Bio E Coulter Awards.
† Major External Funding includes Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) awards, venture capital investment, Initial Public Offerings (IPO) of stock, and other federal or foundation awards supporting development.
For this review, UW CoMotion and the Fred Hutch Office of Business Development and Industry Relations also provided invention disclosure data so that an overall startup rate could be calculated for each institution. The startup rate was calculated by dividing the number of health-related startups by the number of health-related invention disclosures over a 5-year period of time. Data published by the Association of University Technology Managers suggests that a rate of 2.5–3.5 startups per 100 invention disclosures would be a reasonable expectation for institutions of their size and scientific caliber [Reference Jensen and Jones8]. Between 2012 and 2016, the Fred Hutch reported a total of 8 startups and 258 invention disclosures, representing a startup rate of 3.1%, a value much in line with the Association of University Technology Managers predictions. Over the same 5-year period, UW CoMotion reported a total of 49 health-related startups and 916 invention disclosures, representing a 5.4% startup rate (7; CoMotion, personal communication 2017). Between 2008 and 2011, when the DDAC was getting started, and before the CoMotion New Ventures program was implemented, the overall startup rate for the UW School of Medicine was also 3.1%.
Discussion
The DDAC was created by the ITHS to help train and mentor translational researchers seeking to transition their health-related innovations out of discovery and into investigational products ready for human testing and/or commercialization. Unlike other programs that support commercialization, such as the National Science Foundation (NSF)- and National Institutes of Health-funded I-Corps programs [9] or the University of Michigan Early Tech Development Course [Reference Servoss, Chang, Fay and Ward10], the DDAC does not focus on the commercial aspects of early stage inventions, preferring to direct investigators to the Commercialization Fellowship or to the technology transfer offices for support in that area. The DDAC focuses on the preclinical stages of development, with emphasis on refining the clinical indication and the scientific and regulatory requirements related to a specific products’ development. The DDAC consults with investigators at multiple time points, serving as a “virtual” team member to advise investigators on development questions and direct investigators to other resources within the ITHS and community. The DDAC is not a formalized course of instruction.
To inform the biomedical research community of its programs, the ITHS developed a Web site [2] and gave many presentations to faculty conducting biomedical research at the UW and other institutions affiliated with the ITHS through the CTSA and WWAMI programs. Despite these efforts, in its first few years of operation, the number of new translational investigators requesting consultations with the DDAC was lower than expected. At least 3 factors were responsible for the slow uptake of the DDAC program. First, general knowledge of the program was part of a larger marketing challenge for the ITHS. Investigators simply did not know about the program or the ITHS. The ITHS has had to continually refine its messaging in order to reach its core audience. Over time, as the ITHS (and DDAC) became more integrated within the translational community, the refrain of “What is the ITHS” is heard far less often, but marketing of the institute and its programs continues to require constant attention, particularly at institutions located outside of Washington State.
A second factor, perhaps less appreciated initially, was that investigators affiliated with the ITHS, but located at other, non-UW institutions, might have concerns about sharing their unpublished data with the DDAC. As a result, nondisclosure agreements were established with all DDAC members for their participation on the committee. Even so, the number of non-UW investigators consulting with the DDAC remains low, pointing to the challenge of keeping investigators informed of the ITHS and its programs. The ITHS is now introducing “Navigators” in 2 of the ITHS-affiliated institutions, Montana State University and the University of Montana, with the intent of making the ITHS programs more accessible to investigators in those institutions. Washington State University (WSU) (Pullman, WA) has recently asked the DDAC to provide consults for investigators that obtain internal WSU pilot funds.
The final factor, and perhaps the most important, was voiced by several investigators as well as their technology managers. When questioned about their apparent reticence to meet with the DDAC, many felt it was “too early” to engage in discussions about product development. This mindset started to change as more funding programs, both internal and external, started to require investigators to articulate how their proposed research fit into a development plan for the product. Without the knowledge to articulate such a plan, many investigators were unsuccessful in obtaining funding and frustrated by the barrier this represented to their commercialization goals. For this reason, the Ignition Award turned out to be a key catalyst in connecting the DDAC to greater numbers of translational investigators.
As part of the review process, the DDAC reviewers provided detailed critiques to applicants who both succeeded and failed to obtain the awards in hopes that the information would improve their chances for subsequent funding opportunities. These reviews suggested steps that could be immediately taken in an academic setting to strengthen the project and build interest from a grant or investor standpoint. Seeing a potential benefit to their investigators, the technology managers at UW CoMotion started directing investigators to the Ignition Awards and to the DDAC for help in developing preclinical research plans. Over half of those interviewed said that the DDAC consults helped them obtain follow-on funding. This statistic was confirmed with teams that went on to form a startup. Over 50% were successful obtaining funds through the SBIR or STTR awards or through venture capital. At the UW, the integration of the DDAC with the New Ventures program ensured that all recipients of CoMotion Innovation Awards were offered consultation with the DDAC.
Based on investigator feedback, one of the greatest benefits of the DDAC has been the ability to ask project specific questions. This can be seen in the large number of teams that returned to the DDAC on their way to forming a startup. This type of mentoring is possible because of the breadth of expertise on the DDAC (e.g., regulatory, clinical, manufacturing, safety, etc.) and their combined experience bringing different medical products to market. The DDAC mentors were particularly helpful to investigators trying to interpret the Code of Federal Regulations and other FDA regulatory guidance relevant to the processes required or recommended for their product type. For technology managers, the DDAC consults were a cost-effective way of obtaining product-specific regulatory information for their projects. Technology managers did not have to hire external regulatory consultants for all projects and could use scarce funds with more confidence when they had a group like the DDAC to point teams in the right direction. Also, given its focus on preclinical research, the DDAC does not compete with commercialization programs like I-Corps, but complements the information needed to advance the product toward commercialization.
While the “return” metric is helpful in gauging the value of the program to participating investigators, we were also interested in finding out if the DDAC contributed to the overall startup rate for participating institutions. In order to assess this, we compared the startup rates for teams coming out of the UW School of Medicine and the Fred Hutch, 2 ITHS-affiliated organizations that have historically had different DDAC utilization rates. Since faculty quality, federal funding, and number of invention disclosures are significant predictors of the number of startups [Reference Jensen and Jones8], we hypothesized that a lack of impact would result in both organizations having similar commercialization rates and that the number of startups would be proportional to the number of invention disclosures. Instead, we found that the UW startup rate increased from 3.1% (prior to 2012) to 5.4% between 2012 and 2016. During that same 5-year period, the DDAC supported 66% of the UW health-related startups and only 25% of the Fred Hutch startups. We propose that the 85% increase in UW health-related startups is due to the focused resources provided by the CoMotion New Ventures program, which include DDAC consults, market evaluations, and gap funding. Based on feedback from investigators, the DDAC consults were particularly important in obtaining follow-on funding as well as ensuring that the teams were kept informed of new programs that might benefit them, including other gap funds and the summer Fellowship program. Interestingly, 6 of the startups supported by the Fellowship program received commercialization investments from WRF Capital, a partner in the Fellowship program.
Conclusions
This report provides baseline information on the types of programs and services that have been developed by the ITHS to support medical product development. This report also provides baseline information on the ratio of DDAC consults to startups for one large university and affiliated institutions. The ITHS tracked a number of metrics to assess the success of the consulting program. These included follow-on funding, the number of clinical studies initiated, the number of recipients of one or more DDAC programs, the number of teams that formed a startup, and the number of startups that secured follow-on financing from federal or foundation awards or venture capital. Although initiation of clinical studies, including IND and IDE submissions, was an early priority of the program, the return rate and startup rate appear to be more relevant given that the clinical timeline for new drugs and devices is very long and frequently constrained by financial considerations. In the experience of the DDAC, the majority of investigator-initiated clinical studies conducted in academic institutions were not connected with commercialization of a new drug or device, but rather testing of new indications for marketed drugs, or collection of clinical data for in vitro diagnostics.
In conclusion, we recommend a formalized working relationship between preclinical consulting committees and the technology transfer offices of participating institutions in hopes of bringing innovations closer to commercial readiness. These partnerships promise a more efficient use of limited development funds and a more in-depth vetting of the business opportunity and regulatory path to human testing [Reference Rose11]. Our data suggest a model of collaboration based on 3 key elements; mentoring with scientific and regulatory input, gap funding for preclinical proof-of-concept studies, and market research support. We recommend that sites use the data presented here to assess the status of their existing commercialization and regulatory training programs.
Acknowledgments
The authors would like to thank Samantha Ogle from the Buerk Center for Entrepreneurship for her excellent management of the ITHS and WRF Commercialization Summer Fellowship Program. The authors also would like to thank the ITHS and the WRF for their continued financial support of the Fellowships and the UW Primate Center for its continuing support of the Ignition Award. Finally, we thank the permanent and ad hoc members of the DDAC for their long-term commitment and interest in this process. These include Beth Etscheid (WRF), Rodney Ho (UW), Floyd Karp (UW), Peter Korytko (Independent Consultant), H. Denny Liggitt (UW), Lilac Muller (Independent Consultant), Royce Morrison (Independent Consultant), Dirk Smith (Independent Consultant), Richard Senderoff (Independent Consultant), and Katie Sprugel (Independent Consultant). Four of the authors, Drs. Rose, Folger Bruce, Antia, and Wills, are also members of the DDAC.
Financial Support
This project has been funded in whole or in part with Federal funds from the National Center for Research Resources and National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the CTSA Program. The authors are affiliated with the following CTSA-funded institution, UW (UL1TR000423). Other contributing authors are supported by the UW or WRF. The Primate Ignition Award is funded by Washington National Primate Research Center (P51OD010425).






