INTRODUCTION
Boron (B) is an essential trace element, with a narrow range between its phytotoxic limit and the deficient level in the soil (Reisenauer et al. Reference Reisenauer, Walsh, Hoeft, Walsh and Beaton1973). This range depends on the soil type, total B content and age of the plant. Boron plays an important role in flowering and fertilization processes, and in boosting yield and quality of crop produce (Kanwar & Randhawa Reference Kanwar and Randhawa1974). It can be one of the main limiting micronutrients in coarse textured, sandy calcareous soils. It is retained in soils by adsorption onto mineral and humic particles and by forming insoluble precipitates (Couch & Grim Reference Couch and Grim1968; Goldberg & Glaubig Reference Goldberg and Glaubig1985). Boron deficiency can be a major constraint to crop production (Sillanpaa Reference Sillanpaa1982), and has been reported in more than 80 countries and for at least 132 crops over the last 70 years (Shorrocks Reference Shorrocks1997). The deficiency of B has been realized as the second most important micronutrient constraint in crops after that of zinc (Zn) on a global scale. Sillanpaa (Reference Sillanpaa1990) acknowledged that 21% of soils in research investigations conducted worldwide across 14 countries were B deficient. In India, B deficiency affects 33% of soils, particularly alluvial soils (Sakal & Singh Reference Sakal, Singh and Tandon1995; Singh Reference Singh and Alloway2008). Excessive and toxic levels of soil B are reported in semi-arid and arid areas and saline soils with drainage problems (Keren & Bingham Reference Keren and Bingham1985). Boron is distributed in various soil components, including soil solution, organic matter (OM) and minerals. Boron in soil solution is readily available for plant uptake, but maintaining B in the soil solution is an important aspect for plant nutrition and it is controlled by the pools of B in other soil fractions and their equilibration with soil solution (Xu et al. Reference Xu, Wang, Bell, Yang and Huang2001). The proportion of various B forms varies considerably, depending on soil type and extraction technique used (Raza et al. Reference Raza, Mermut, Schoenau and Malhi2002). Boron in soil is considered to be distributed among several geochemical forms that include readily soluble, specifically adsorbed, oxide bound, organically bound and residual forms. The proportion of B associated with different geochemical forms depends greatly upon physico-chemical properties of soils, climate and management practices. Several soil factors such as pH, OM, clay minerals, iron (Fe) and aluminium (Al) oxides, carbonates and tillage may influence the content of extractable B and transformations among different soil B fractions (Jin et al. Reference Jin, Martens and Zelazny1987; Mandal et al. Reference Mandal, Adhikari and De1993; Hou et al. Reference Hou, Evans and Spiers1994; Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994; Yermiyahu et al. Reference Yermiyahu, Keren and Chen1995). These B fractions have remarkable differences in mobility, bioavailability and chemical behaviour of soils and can be transformed under certain conditions.
Fractionation is used to identify and quantify various forms of soil B, each forming a fraction of total soil B. Such information is potentially valuable for predicting bioavailability, leaching, dynamics, transformation between various chemical forms in soils and environmental impacts (Hedley et al. Reference Hedley, Stewart and Chauhan1982; Sui et al. Reference Sui, Thompson and Mize1999). Fractionation along with information about the chemistry of B is fundamental for understanding its chemistry in soils and the potential contribution of these fractions to plant uptake of B. Studying the forms of B present in soil provides knowledge of B status and potential supply to plants. Various researchers have quantified total soil B in terms of the following five fractions: (i) readily soluble, (ii) specifically bound, (iii) organically bound, (iv) oxide bound and (v) residual (Jin et al. Reference Jin, Martens and Zelazny1987; Hou et al. Reference Hou, Evans and Spiers1994, Reference Hou, Evans and Spiers1996).
FRACTIONS OF BORON
Readily soluble boron
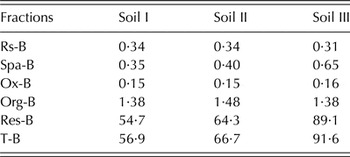
Among all fractions, readily soluble boron (Rs-B) is the first and most readily available form for plant uptake, which is in soil solution or weakly adsorbed by soil particles (Keren et al. Reference Keren, Bingham and Rhoades1985). This pool constitutes 1–2% of total soil B (Jin et al. Reference Jin, Martens and Zelazny1987; Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994). Padbhushan & Kumar (Reference Padbhushan and Kumar2015a ) reported Rs-B forms to be <1% of total B in alkaline calcareous soils of Punjab, India (Table 1). This fraction decreases with increasing amounts of calcium carbonate (CaCO3) in loamy textured soils. Maintaining B in the soil solution is important for plant nutrition (Keren & Bingham Reference Keren and Bingham1985; Keren et al. Reference Keren, Bingham and Rhoades1985) and it is controlled by the B in other soil fractions and their equilibrium with soil solution B.
Table 1. Estimate of boron (B) fractions in different calcareous soils (Soil I – 7·5, Soil II – 21·0 and Soil III – 47·5 calcium carbonate g/kg soil) of Punjab, India (mg B/kg soil)

Rs-B, readily soluble boron; Spa-B, specifically adsorbed B; Ox-B, oxide bound B; Org-B, organically bound B; Res-B, residual B; T-B, total B.
Specifically adsorbed boron
The specifically adsorbed boron (Spa-B) fraction may be specifically adsorbed onto clay surfaces or associated with OM in soil (Jin et al. Reference Jin, Martens and Zelazny1987), and depends mainly upon the clay content of the soil. It is the other fraction, after Rs-B, which is available for plant uptake (Jin et al. Reference Jin, Martens and Zelazny1987, Reference Jin, Martens and Zelazny1988; Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994). This pool constitutes 0·01–0·61% of total soil B (Xu et al. Reference Xu, Wang, Bell, Yang and Huang2001). Similar amounts of the fraction were reported by Padbhushan & Kumar (Reference Padbhushan and Kumar2015a ) in the alkaline calcareous soils of Punjab (Table 1); these authors found that the amount increased slightly on soil B application. Boron adsorption occurs on the clay edges of illite (Couch & Grim Reference Couch and Grim1968) and montmorillonite (Keren et al. Reference Keren, Gast and Bar-Yosef1981). Illite has a greater proportion of edge surface area than montmorillonite.
Oxide-bound boron
The oxide bound boron (Ox-B) fraction is associated with oxides and hydroxides of Fe and Al. Manganese (Mn) forms a part of the structure through isomorphous substitution (McLaren & Crawford Reference McLaren and Crawford1973; Tessier et al. Reference Tessier, Cambell and Bisson1979; Shuman Reference Shuman1985). It is a less labile fraction of B, which sorbs B into unavailable forms (Jin et al. Reference Jin, Martens and Zelazny1988). Boron sorption behaviour in soils has indicated that Al and Fe oxide minerals play an important role (Harada & Tamai Reference Harada and Tamai1968; Bingham et al. Reference Bingham, Page, Coleman and Flach1971; Elrashidi & O'Connor Reference Elrashidi and O'Connor1982). This pool constitutes <3% of total boron (T-B) (Hou et al. Reference Hou, Evans and Spiers1994).
Organically bound boron
Hou et al. (Reference Hou, Evans and Spiers1994, Reference Hou, Evans and Spiers1996) included OM-bound forms in their proposed B fractionation procedure. The organically bound fraction is bound to several forms of OM (detritus, organic coatings on mineral particles and humus bound fraction). Organic matter adsorbs B (Goldberg Reference Goldberg1997), which is then unavailable for plant uptake (Hou et al. Reference Hou, Evans and Spiers1994). This fraction constitutes 2–8% of T-B.
Residual boron
The largest proportion among all fractions of B is composed of residual boron (Res-B) and it does not relate with plant available B (Jin et al. Reference Jin, Martens and Zelazny1987; Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994; Padbhushan & Kumar Reference Padbhushan and Kumar2015b ). Residual B is associated with primary and secondary minerals within the crystal structure and constitutes a substantial portion of T-B that is unlikely to be released in the mid- and long-term under the conditions normally found in native soils (McLaren & Crawford Reference McLaren and Crawford1973; Tessier et al. Reference Tessier, Cambell and Bisson1979; Shuman Reference Shuman1985; Chao & Sanzolone Reference Chao and Sanzolone1989). The residual fraction is a non-labile form of B. This pool constitutes about 87·4–99·7% of T-B.
Total boron
The T-B concentration in soil varies according to its parent material and degree of weathering (Barber Reference Barber1995): the range of T-B in agricultural soils is 2–100 mg/kg (Swaine Reference Swaine1955). In most Indian soils, the content has been found to vary between 3·8 and 630 mg/kg (Takkar Reference Takkar, Randhawa, Goswami, Prihar, Abrol, Murthy, Krishna Murti, Singh, Ghosh, Sastry and Narayanasamy1982). Hadwani et al. (Reference Hadwani, Gandhi, Patel and Yadav1989) observed that the total B content decreased with soil depth in all profiles. The T-B content of a soil is not a reliable indicator of available B for plant uptake (Nable et al. Reference Nable, Banuelos and Paull1997).
RELATIVE PROPORTION OF BORON FRACTIONS
The relative proportion of various B fractions in soils depends upon various factors, especially inherent properties as well as soil application of B fertilizers. The parent material, texture, OM content, clay content and the microbial activities determine the extent of particular B fractions in the soil. Residual B constitutes the major proportion of T-B, which is not available for plant uptake. Hou et al. (Reference Hou, Evans and Spiers1994) reported that Res-B accounts for about 99% of T-B in soils of Ontario. Xu et al. (Reference Xu, Wang, Bell, Yang and Huang2001) observed that non-specifically adsorbed boron (Nsa-B), Spa-B and Ox-B comprised 0·06–0·99, 0·01–0·61 and 0·03–7·57% of T-B, respectively. Raza et al. (Reference Raza, Mermut, Schoenau and Malhi2002) studied nine composite surface soil samples collected from south-western and north-eastern agricultural regions of Saskatchewan and found that the mean contents of extractable B were 1·02% of the total B in Rs-B, 0·69% in Spa-B, 0·40% in Ox-B and 0·86% in organically bound boron (Org-B). About 92–97% of T-B was in Res-B or occluded fraction. Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002) collected 17 surface soil samples from different locations of India and reported that T-B ranged from 31 to 355 mg/kg. The Rs-B, Spa-B, Ox-B and Org-B constituted 0·38, 0·34, 1·45 and 0·62% of T-B, respectively. Residual B accounted for the major portion of T-B with a mean of 97·1%. Padbhushan & Kumar (Reference Padbhushan and Kumar2015b ) reported that T-B in soil samples collected from Punjab, India, with CaCO3 contents of 7·5, 21·0 and 45·6 g/kg soil, were 56·9, 66·7 and 91·6 mg/kg, respectively. The Rs-B in these soils varied from 0·60, 0·50 and 0·34% of T-B for these. It accounted for <1% of T-B. Specifically adsorbed B was 0·61, 0·60 and 0·71%, Ox-B was 0·26, 0·23 and 0·16%, Org-B was 2·42, 2·22 and 1·51% and Res-B was 96·1, 96·4 and 97·3% of the T-B for the three soils, respectively. Overall, the contents of B followed the order of Res B > Org-B > Spa-B > Rs-B > Ox-B. The extent of all fractions may vary depending upon the extent of influencing factors in the soils.
FACTORS INFLUENCING BORON FRACTIONS IN SOIL
Climate
Climate is the predominant factor affecting soil properties and B fractions. Increased rainfall and temperature increases the adsorption of B as the Fe–Al oxides and oxyhydroxides and the Mn-oxyhydroxides that will contribute to declining extractable B levels. This is due to the ligand exchange phenomenon, as suggested by Bingham et al. (Reference Bingham, Page, Coleman and Flach1971). Xu et al. (Reference Xu, Wang, Bell, Yang and Huang2001) explained that decline in B content is correlated with increasing rainfall; this may be attributed in part to prolonged leaching losses of B in soils that receive high precipitation. However, the content of mineral fractions in the soils that adsorb B, such as the amorphous Fe–Al oxides and oxyhydroxides and the Mn-oxyhydroxides, also increase with increasing precipitation, and this will also contribute to declining extractable B levels. The distribution of B in various fractions was also related to site rainfall and temperature. The content of Nsa-B fraction significantly decreased with increasing mean annual rainfall (Xu et al. Reference Xu, Wang, Bell, Yang and Huang2001). This is due to stronger B leaching regime in the regions with more abundant rainfall. No clear relationship has been documented between Spa-B fraction and climate. A negative correlation has been recorded between Ox-B and rainfall and temperature (Xu et al. Reference Xu, Wang, Bell, Yang and Huang2001). No significant correlation has been observed between Res-B and climate factors.
Soil pH
Soil pH is one of the important factors, which influence the extent of many fractions of B in soils. The content of water-soluble B in soils tends to increase with soil pH, but not always in a consistent manner (Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994), because B adsorption by soil components also increases with the increase in soil pH and reaches a maximum in the alkaline pH range (Gu & Lowe Reference Gu and Lowe1990; Goldberg et al. Reference Goldberg, Forster and Heick1993, Reference Goldberg, Forster, Lesch and Heick1996). The content of Nsa-B fraction has been shown to increase significantly with increasing pH. Tsalidas et al. (Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994) found that the Spa-B fraction was correlated with soil pH. No significant correlation was observed between Ox-B, Org-B, Res-B and soil pH. Hou et al. (Reference Hou, Evans and Spiers1994) carried out sequential fractionation of B in mineral and synthetic soils and recorded that Rs-B was positively correlated with pH. Goldberg (Reference Goldberg1997) reported that adsorption of B exhibited a peak around pH 8–9. Xu et al. (Reference Xu, Wang, Bell, Yang and Huang2001) observed that Nsa-B increases significantly with increasing pH. No significant relations with other fractions of B were observed. Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002) reported in Indian soils that the Rs-B fraction has a positive relationship with pH (Table 2). The positive effect of soil pH on Rs-B is ascribed to the fact that increasing pH increases the negative surface charges of clays and other variable surface charges (Hingston Reference Hingston1964). Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002) also reported that the amount of Rs-B was approximately the same up to pH 7·0, and beyond this pH range a spectacular increase occurred (Table 2). Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002) did not find any relation between pH and other B fractions. The occurrence of Rs-B in the soils is correlated with soil pH, while other fractions are not.
Table 2. Simple correlation coefficients (r) between boron fractions and physicochemical properties of different soils of India

OC, organic carbon; Al, aluminium; Fe, iron; Rs-B, readily soluble boron; Spa-B, specifically adsorbed boron; Ox-B, oxide-bound boron; Org-B, organically bound boron; Res-B, residual B.
* NH4-oxalate extractable (pH 3·25).
Soil organic matter
Organic matter plays an important role in adsorption and desorption of B: soil organic matter (SOM) adsorbs more B than mineral soil constituents (Yermiyaho et al. Reference Yermiyaho, Keren and Chen1988; Gu & Lowe Reference Gu and Lowe1990). A possible mechanism for B sorption by OM is ligand exchange (Yermiyaho et al. Reference Yermiyaho, Keren and Chen1988). However, Jin et al. (Reference Jin, Martens and Zelazny1987) found no significant correlation between Spa-B and SOM. Hou et al. (Reference Hou, Evans and Spiers1996) reported that OM plays an important role in determination of fractions of B and proposed a method for extraction of Org-B; although the scientific community was slow to recognize this, it was later accepted that OM does influence Org-B. A significant correlation was observed between Org-B and OM while no significant correlation was observed between Rs-B, Spa-B, Ox-B, Res-B and OM (Datta et al. Reference Datta, Rattan, Suribabu and Datta2002) (Table 2). A significant positive correlation between Org-B and OM was observed in different calcareous soils of India, Punjab (Padbhushan & Kumar Reference Padbhushan and Kumar2015a ). This may be because Org-B binds with OM and any increase in the content in OM increases the Org-B in soils.
Clay content
Clay plays a major role in B sorption, both in terms of quantity of clay and the type of clay. The magnitude of B sorption in soil is greater in soils with greater clay fractions and vice versa (Xu et al. Reference Xu, Wang, Bell, Yang and Huang2001). As the clay content increases in the soil, the sorption of B also increases. Boron availability depends upon the nature of clay present in the soil. Illite has greater clay edge surface area compared with montomorillonite (Couch & Grim Reference Couch and Grim1968; Keren et al. Reference Keren, Gast and Bar-Yosef1981). Thus, B sorption is greater in illitic soil, as observed in the soils of Indian Punjab (Padbhushan & Kumar Reference Padbhushan and Kumar2015a ). Jin et al. (Reference Jin, Martens and Zelazny1987) reported a positive correlation between Spa-B and clay content, while Tsalidas et al. (Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994) found that Spa-B was not correlated with clay content. Similarly, Xu et al. (Reference Xu, Wang, Bell, Yang and Huang2001) was not able to observe any relationship between B fractions and clay content in Chinese soils. Although a significant correlation is expected between the clay content of the soils and the Spa-B, the literature must be critically examined where the correlation is not observed. This could happen when the soils involved in the correlation study are similar in terms of clay content, or when illite is not the dominant clay mineral, or even when the content of illite is similar among the soils studied. Although Hou et al. (Reference Hou, Evans and Spiers1996) reported that in addition to Spa-B, Res-B content was also correlated significantly with clay content.
In general, Spa-B and Org-B are positively correlated with clay content in soil. Residual B was also found to be significantly and positively correlated with clay content, as Res-B is the structural constituent of clay. A similar correlation for Res-B and clay was reported by Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002). No significant correlation was observed between Rs-B and Ox-B (Table 2). Padbhushan & Kumar (Reference Padbhushan and Kumar2015a ) observed a significant positive correlation between Ox-B, Res-B and clay content. This was due to an increase in clay content, which increases the surface area of the soil resulting in more Ox-B in the soil; also because Res-B is the structural constituent of clay, which ultimately results in an increase in the amount of Res-B with an increase in the clay content of soil.
Ammonium-oxalate extractable iron and aluminium
Hou et al. (Reference Hou, Evans and Spiers1994) and Datta et al. Reference Datta, Rattan, Suribabu and Datta2002 failed to establish a positive relationship between the Spa-B and ammonium (NH4)-oxalate extractable Fe and Al. This fraction probably originates from the weak binding site of both organic and inorganic constituents. However, it was later observed that the NH4-oxalate extractable Fe and Al increased with increasing precipitation and contributed to declining extractable B levels (Xu et al. Reference Xu, Wang, Bell, Yang and Huang2001). A significant correlation was observed between Ox-B, Org-B, Res-B and NH4-oxalate extractable Fe and Al. This is due to the acidic dissolution and/or complexation mechanism, while no significant correlation was observed with Rs-B and Spa-B (Datta et al. Reference Datta, Rattan, Suribabu and Datta2002) (Table 2).
Calcium carbonate
No significant correlation has been found between CaCO3 and water-soluble fraction of soils (Bhattacharjee Reference Bhattacharjee1956; Gandhi & Mehta Reference Gandhi and Mehta1958; Mathur et al. Reference Mathur, Moghe and Talati1964; Grewal et al. Reference Grewal, Randhawa and Bhumbla1969; Singh & Randhawa Reference Singh and Randhawa1977). However, Paliwal & Mehta (Reference Paliwal and Mehta1973) found that in the soils of Kota and Bhilwara regions of Rajasthan, water-soluble B was negatively related to CaCO3 content (r = −0·63, P < 0·01), and Elseewi & Elmalky (Reference Elseewi and Elmalky1979) found that the acid-soluble fraction of B was negatively related with CaCO3 content of the soil. Sims & Bingham (Reference Sims and Bingham1968) threw considerable light on the nature of B retention and provided a better understanding of the reactions involved in B fixation. As the main reaction occurs when acid soils are limed, the replacement (by calcium) of exchangeable Al, and hydroxyl-Al cations, occurs with their resulting precipitation as aluminium hydroxide (Al(OH)3). The adsorption of B on Al(OH)3 was studied by Hatcher et al. (Reference Hatcher, Bower and Clark1967), who demonstrated that freshly precipitated Al(OH)3 adsorbed large quantities of B, while adsorption decreased markedly with time. The reaction between exchangeable Al and lime may be summarized as follows:
where X is the exchange site. The freshly precipitated Al(OH)3 is then available for adsorption of B. The CaCO3 content of salt-affected soils in Punjab was found to be significantly correlated with the available and T-B (Sharma Reference Sharma1984).
RELATIONSHIP BETWEEN BORON FRACTIONS
To understand the dynamics, transformation and bioavailability of B, interactions between various B fractions are important. Interactions between various B fractions determine the status of the fractions resulting from uptake of B at later stages of crop growth. The readily soluble fraction quantity is reduced in the later stages of crop growth, leading to a belief that it might be converted into other fractions of B. Tsalidas et al. (Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994) carried out work in Greek soils and reported no correlation between any of the B fractions. Hou et al. (Reference Hou, Evans and Spiers1996) investigated interaction between B fractions and observed that Nsa-B had a close correlation with oxide- and hydroxide-bound B. Xu et al. (Reference Xu, Wang, Bell, Yang and Huang2001) observed the interaction between the various B fractions in Chinese soils and concluded that Nsa-B was positively correlated with oxide B forms such as Moh-B and B occluded in crystalline Fe and Al oxides (Cro-B) fractions. There was also a close relationship between Spa-B and Moh-B (Table 3), and Spa-B was negatively and significantly correlated with Rs-B (Padbhushan & Kumar Reference Padbhushan and Kumar2015a ). This showed that as the time passed, the readily soluble fraction transformed into Spa-B, while no significant relation was observed with other B fractions. To date, very few investigations have been carried out into the relationships between B fractions. More studies are needed to determine the dynamics, transformation and bioavailability of B. Similarly, more studies on interactions between the different fractions of B in different soils with B fertilization are needed.
Table 3. Correlation coefficients (r) between various soil boron (B) fractions for Chinese soil

Hws-B, Hot water-soluble B; Nsa-B, non-specifically adsorbed B; Spa-B, specifically adsorbed B; Moh-B, B occluded in Mn-oxyhydroxides; Amo-B, B occluded in amorphous Fe and Al oxides; Cro-B, B occluded in crystalline Fe and Al oxides; Res-B, residual B.
RELATIONSHIP OF EXTRACTABLE BORON WITH BORON FRACTIONS
Jin et al. (Reference Jin, Martens and Zelazny1987) compared the B fractions with various extractants in 14 temperate soils and presented different proportions of B: they reported that calcium chloride (CaCl2) as an extractant has a high proportion of Nsa-B. Similar results were reported by Badr-Uz-Zaman & Salim (Reference Badr-Uz-Zaman and Salim1999), who studied eight soils in Pakistan and reported that little B extracted by CaCl2 was in non-specifically adsorbed form. The proportion of Nsa-B ranged from 0·4 to 2·3% with an average value of 1·2%. The Spa-B, from the soil extracted with mannitol, was 14·2–19·2% of total soil B with a mean content of 17·4%. Raza et al. (Reference Raza, Mermut, Schoenau and Malhi2002) reported that an average of 1% of the total soil B was present as hot water-soluble B. The 0·01 m CaCl2 and 1 m NH4-acetate extracted 0·74 and 0·37% of the total soil B, respectively. The major portion of soil B existed in the residual or occluded form. This was also reported when 0·01 m CaCl2, 1 m NH4 acetate and anion exchange membranes were used as Rs-B extractants.
Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002) observed that hot CaCl2 extracted the highest amount of B followed by salicylic acid, hydrochloric acid, tartaric acid, NH4 acetate (NH4OAc) at pH 4·8, CaCl2 + mannitol and NH4OAc (pH 7·0). Stepwise regression indicates that 75% of the variability in hot CaCl2 extractable B (Hcc-B) could be attributed to Rs-B, Spa-B, Ox-B and Org-B. The Spa-B and Org-B fractions could explain 80% of the variability in salicylic acid extractable B (Sa-B), whereas Rs-B and Spa-B fractions could account for 64% of the variability in CaCl2 + mannitol extractable-B (Caml-B). Specifically adsorbed B accounted for 56% of the variation for NH4OAc (pH 4·8) extractable-B, while Rs-B and Res-B could explain 48% of the variation in HCl extractable-B (HCl-B). Residual B, Spa-B and Org-B could explain 65% of the variation in tartaric acid extractable-B (Ta-B). Since Hcc-B and Sa-B extractants isolate the highest amount of B from the soil samples, they are more capable of extracting Rs-B, Spa-B and Org-B fractions and are also better predictors of plant available B in soils (Table 3). The extractable B was found to be positively related with organic carbon (Mandal et al. Reference Mandal, Padbhushan, Kumar, Vimal, Das, Bhowmick and Kumar2016).
FRACTIONATION TECHNIQUE FOR SOIL BORON
Various fractionation techniques have been developed for soil B using methods originally developed for selective dissolution of trace metals, in which a particular fraction of the element might be removed by a specific extractant. Jin et al. (Reference Jin, Martens and Zelazny1987) developed a separate extraction scheme to determine the distribution of B between different fractions in soils. Hou et al. (Reference Hou, Evans and Spiers1994, Reference Hou, Evans and Spiers1996) modified this scheme and developed a sequential fractionation method for partitioning total soil B among different distinct pools. Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002) proposed colorimetric method to determine the various forms of B and later Padbhushan & Kumar (Reference Padbhushan and Kumar2015a ) developed the procedure using combined colorimetric (Rs-B and Spa-B) and ICAP-AES (inductively coupled plasma atomic emission spectroscopy) (Ox-B, Org-B and Res-B) to estimate the different B forms. A flow diagram for fractionation of B in soils as proposed by Datta et al. (Reference Datta, Rattan, Suribabu and Datta2002) and Padbhushan & Kumar (Reference Padbhushan and Kumar2015a ) is presented in Fig. 1.

Fig. 1. Flow diagram for the fractionation of boron in soils.
Another sequential extraction scheme for B in soils and sediments is shown in Hou et al. (Reference Hou, Evans and Spiers1996) for synthetic and mineral soil.
PLANT RESPONSE
Hot water-extractable B has been regarded as a suitable index of plant-available B (Bingham Reference Bingham, Page, Miller and Keeney1982), but in some studies, levels of hot water-extractable B have not been correlated with plant response (Sims & Johnson Reference Sims, Johnson, Mortvedt, Cox, Shuman and Welch1991), suggesting that a better understanding is needed of the pools of soil B accessed by prevailing soil B tests and their relationship to plant B uptake (Bell Reference Bell1997). Moreover, the available forms of soil B vary with plant species (Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994). Jin et al. (Reference Jin, Martens and Zelazny1987) found that B concentration in maize tissue correlated positively not only with Ws-B, but also with Nsa-B, Spa-B and Mho-B. Tsalidas et al. (Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994) showed that B content in olive tree (Olea europaea L.) leaves correlated well with amorphous Fe–Al oxyhydroxide-occluded B, Spa-B and Mho-B besides WS-B. By contrast, in barley (Hordeum vulgare L.) leaves B content was correlated with Nsa-B as well as amorphous Fe–Al oxyhydroxide-occluded B, Spa-B and WS-B, but not with Mn-oxyhydroxide-occluded B (Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994). It remains to be demonstrated that the Mn-reducible and Org-B fractions can be clearly distinguished and such a distinction is important for predicting plant response to soil B.
Jin et al. (Reference Jin, Martens and Zelazny1988) concluded that oxyhydroxides of Al and Fe sorb B into unavailable forms. Thus, the Nsa-B, Spa-B and Moh-B fractions may be most readily available to plants (Jin et al. Reference Jin, Martens and Zelazny1987, Reference Jin, Martens and Zelazny1988; Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994), although this needs further investigation. In 13 Chinese soils examined, 87·4–99·7% of soil B was in the residual fraction (Res-B), which generally does not relate well to plant-available B (Jin et al. Reference Jin, Martens and Zelazny1987; Tsalidas et al. Reference Tsalidas, Yassoglou, Kosmas and Kallianou1994). Therefore, a better understanding of the distribution of B in various soil fractions and their relationships with plant response would provide a basis for assessing the availability of soil B to plants and formulating field management practices to influence B availability. This need is especially evident in areas where soils contain low levels of B and deficiency is a significant limit to crop production (Shorrocks Reference Shorrocks1997).
For a green gram crop sown in B-deficient calcareous soils of Punjab (Padbhushan & Kumar Reference Padbhushan and Kumar2015b ), at grand growth stage, a significant increase in Rs-B with B applications was recorded while at maturity there was a significant increase only up to a certain level of B application (0·75 mg B/kg). Readily soluble fractions decreased at maturity in comparison with the grand growth stage at different levels of soil applied B. This might be due to increased B uptake and transformation of readily soluble fraction to oxide bound and organically bound fraction at maturity. In the case of Spa-B at grand growth stage, there was no significant increase at any level of B application as compared with the control, while at maturity there was a significant increase only up to 0·75 mg B/kg. In Ox-B at both stages of crop growth, there was a significant increase at all levels of B application. In Org-B at both stages of crop growth, there was no significant increase at any levels of B application as compared with the control. However, an increase in organically bound at maturity from grand growth stage was observed, numerically. This might be due to more OM addition in the soil. Total B decreased at maturity as compared with grand growth stage in green gram crop, which may be due to increase in B uptake by green gram crop from grand growth to maturity stage (Padbhushan & Kumar Reference Padbhushan and Kumar2015b ).
Boron application and soil type have a significant interactive effect on shoot dry weight. Widespread responses to B application on alkaline calcareous soils may be due to an increased B requirement by agricultural crops due to a widened Ca/B ratio in the soil–plant system (Fleming Reference Fleming and Davis1980; Tisdale et al. Reference Tisdale, Nelson and Beaton1985).
FUTURE STUDIES
In order to better understand the dynamics, transformation and bioavailability of B fractions, further studies on interactions between the different fractions in various soils with application of B fertilizers should be carried out. Relationships between various B fractions and their interactions with crop growth need to be understood for various soils.
CONCLUSION
Boron fractionation of soils provides insights for qualitative and quantitative significance of B fractions. In general, Res-B forms the major fraction of T-B followed by Org-B, Spa-B, Rs-B and Ox-B, respectively. Residual B is significantly correlated with pH, climatic factors and CaCO3 content in soils, Spa-B is significantly correlated with clay content of the soil, Ox-B is significantly correlated with climatic factor, NH4 +-oxalate extractable Fe and Al and Org-B is significantly correlated with OM content in soil. Among all fractions, Rs-B is the most available form for plant uptake. Transformation of Rs-B to different fractions of B takes place at maturity of crop. Nsa-B, Spa-B and Moh-B are found responsive to B uptake in plant.