Introduction
Efforts to improve the environmental sustainability of healthcare systems have been gaining pace rapidly, at both national and international levels. At the recent COP28 UN Climate Change Conference, 143 countries committed to the urgency of taking action on climate change to improve health and the need to transform health systems “to be climate-resilient, low-carbon, sustainable and equitable” (1). The World Health Organization has recently produced important guidance on climate-resilient and environmentally sustainable health systems and facilities (2), and a growing number of nations have developed national strategies in this area. While much of the focus on “sustainability” has understandably been driven by recognition of the increasingly urgent need for action on decarbonization and climate change, most strategies clearly recognize the importance of other aspects of environmental sustainability beyond climate change (3) and the broad-spectrum ecological crisis that now sees human impacts pushing outside the safe operating spaces for six out of nine “planetary boundaries” (Reference Richardson, Steffen and Lucht4). Unsurprisingly, health technology assessment (HTA) agencies, researchers, and industry have also become increasingly interested in these sustainability challenges and in whether and how best to incorporate them within HTA processes (Reference Desterbecq and Tubeuf5–Reference Polisena, De Angelis, Kaunelis, Shaheen and Gutierrez-Ibarluzea8). Yet much of the discussion of how to include environmental impacts within HTA evaluation has tended to move very quickly to methodological issues (e.g. are environmental impacts costs or outcomes; what are the best measures to use; should all HTAs include a life cycle analysis of environmental impacts? etc.) and has not focused on the relative contribution of HTA within the wider array of sustainability policy levers and instruments available at whole-system level (Reference Pekarsky9;Reference Hensher10). This Perspective seeks to step back and consider those ways in which HTA can most cost-effectively contribute to improving healthcare environmental sustainability and those areas where other policy levers are likely to be more effective.
Healthcare sustainability: System-level aims and levers
WHO defines climate-resilient and low-carbon health systems “as those capable of anticipating, responding to, coping with, recovering from, and adapting to climate-related shocks and stress, while minimizing GHG [greenhouse gas] emissions and other negative environmental impacts to deliver quality care and protect the health and well-being of present and future generations” (2). This Operational Framework starts from WHO’s longstanding six building blocks of health systems (11): leadership and governance, health workforce, health information systems, essential medical products and technologies, service delivery, and financing. The Framework then maps a range of key action areas against each building block. In the case of medical products and technologies (the purview of HTA), the Framework calls for “climate resilient and low carbon infrastructures, technologies and supply chain” (2). This is a helpful starting point for a discussion of the role of HTA within this specific ecosystem.
In practice, most HTA agencies and processes are primarily concerned with undertaking assessments of new technologies – the flow of new innovations into the health system (Figure 1). Yet, by definition, almost all of the current environmental impacts (both GHG and others) are driven by the existing stock of technologies and infrastructure already in place (and the extant product base and supply chains that support them). But the pipeline of new technologies will, of course, drive future emissions and environmental impacts. Given the very rapid rate of reduction in GHG emissions needed to mitigate catastrophic climate outcomes, effective policy action to create climate-resilient and low-impact health systems requires urgent action on both the existing technology stock and on the new technologies pipeline.

Figure 1. HTA, new technology flow, and the existing system stock.
The potential role of HTA in mitigating the environmental impacts of new technology adoption by the healthcare system is clear and directly analogous to its existing roles in terms of costs and consequences. Yet HTA has long been criticized for its practical failure to address “technology management” of existing technologies already in use (Reference Bryan, Mitton and Donaldson12); simply adding environmental impacts to HTA processes should not, in itself, be expected to change HTA’s limited impact on already deployed technologies. Fortunately, a wide range of other policy tools and levers exist that can potentially be used in parallel with (or perhaps in place of) HTA, to act on both the demand for and supply of new and existing technologies (Reference Hensher13). Potential mechanisms of relevance to environmental mitigation and/or climate resilience for health technologies might include initial licensing requirements; direct regulations and standards targeting manufacturers/suppliers and/or healthcare providers; procurement standards and requirements (e.g. the NHS “net zero supplier roadmap”) (14); subsidies or taxes, either domestic or on imports (e.g. extension of the European Carbon Border Adjustment Mechanism) (15); financial or behavioral incentives to modify provider or patient choices and actions; and through the broader impacts of wider policy choices (e.g. the impact on healthcare of a wider societal choice to decarbonize electricity generation). The key question (the answer to which will differ from country to country) must therefore be: what is the optimal mix of these policies to deliver a climate-resilient, low-environmental-footprint healthcare system and how and where can HTA best contribute within this policy mix?
The relative significance of HTA as an effective contributor to overall health system sustainability will therefore depend upon a number of factors. The scale and impact of the HTA/new technology pipeline relative to that of the existing healthcare system technology stock is clearly significant, as is the level of challenge or capability to rapidly and effectively mitigate the environmental impacts of existing system infrastructure of technologies (e.g. if the environmental impact of current technologies cannot be effectively mitigated, then their replacement with new alternatives will become relatively more important and urgent). The ability of policymakers to influence supply chains and supplier decisions using multiple policy instruments is clearly of importance; for example, a small, highly import-dependent jurisdiction may have limited leverage on supply chains, while a large market such as the European Union might have very substantial leverage. Finally, the availability or scarcity of the skills and capability to undertake environmental assessments (e.g. life cycle analysis) may be a significant rate-limiting factor constraining the ability of HTA systems to undertake the work necessary to incorporate environmental impacts routinely within HTA. Taken together, this suggests that careful thought must be given to constructing the most effective possible mix of policies, and particular attention must be paid to prioritizing scarce technical resources (e.g. LCA, environmental input–output analysis) strictly toward those areas where they can have the most impact. The rest of this paper suggests a possible approach to achieving this latter aim.
Intrinsic versus generic environmental impacts
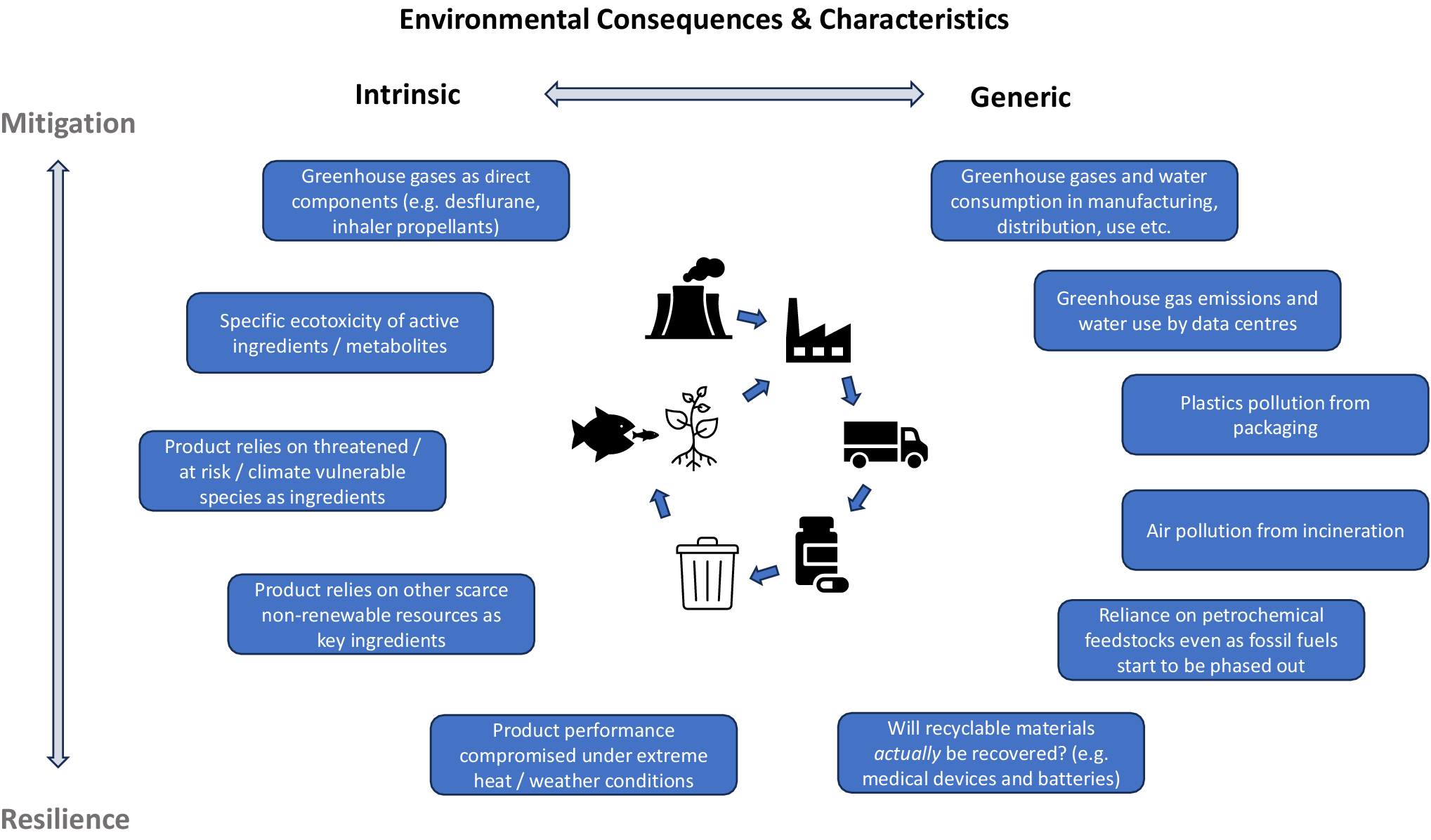
All healthcare products have an environmental footprint resulting from their production, distribution, use, and disposal. We are all by now familiar with certain products that have very significant environmental impacts, such as the extremely high carbon footprints of the greenhouse gases used as propellants in pMDI inhalers, or of the anesthetic gases desflurane and nitrous oxide – whose impacts relate to the very nature of certain constituent chemicals in these products. Yet most other medical products in fact have quite similar – we might say “generic” – environmental impacts relating primarily to energy use, carbon footprint, and other pollution consequences of the standard manufacturing, transport, usage, and disposal processes common to many product categories. We might therefore distinguish between the intrinsic environmental impacts of a specific product – any unique or especially significant environmental impacts intrinsic to this product; or those common environmental impacts of a product that are largely generic consequences of standard processes (e.g. fossil fuel use in the product’s manufacture and transport). Figure 2 illustrates examples of these categories of intrinsic or generic environmental consequences for both mitigation and resilience.

Figure 2. Intrinsic versus generic environmental consequences and characteristics of health technologies.
Figure 2 illustrates this spectrum of intrinsic versus generic environmental consequences and characteristics, potentially providing a basis for differentiating between those products that might carry elevated risks of particular product-specific harms from those for which these impacts are likely to be shared with many other products. Broadly speaking, given the currently strictly limited availability of skilled personnel able to undertake LCA and other relevant environmental assessments for healthcare products, I would suggest that their inclusion in product-by-product HTA processes is likely to yield significantly greater value if focused on those products with a high likelihood of possessing significant intrinsic environmental consequences. By contrast, the much larger group of products whose environmental consequences are primarily generic in nature would be more cost-effectively dealt with via appropriate generic analysis and policy instruments targeting these general mechanisms of impact. Such an approach requires two critical elements to be in place: (1) an effective environmental screening assessment to identify early on any intrinsic impact characteristics of products (Reference Hensher10), and (2) genuine integration of HTA environmental assessment processes within a wider armamentarium of effective policy measures to control generic environmental impacts.
Figure 2 also illustrates the increasing importance of resilience for HTA, especially as climate change impacts themselves increasingly become felt in supply chains (see Box 1). Adopting healthcare technologies that have unacceptably risky dependencies on vulnerable key ingredients will increasingly undermine health system resilience, as well as potentially accelerate biodiversity loss and ecological damage through overexploitation. Yet the incorporation of resilience within HTA poses challenges – especially because there are, as yet, no simple metrics for “resilience,” implying a need for more qualitative, risk-based assessments.
Box 1: Quillaja Saponaria and healthcare system resilience
While it has been used as an adjuvant in animal vaccines for decades, saponin bark extract from the Chilean Soaptree (Quillaja Saponaria) has more recently been approved as an adjuvant in a number of human vaccines, including shingles and malaria (Reference Wang16); with high hopes, it may also be an effective adjuvant for TB vaccines (Reference Gyu Choi, Woong Kwon and Jae Shin17). The supply of Quillaja Saponaria bark extract is limited; the tree is grown only in a relatively limited region of Chile and has been viewed as being at risk of overexploitation (with many different economic applications) for years (Reference San Martin and Briones18). Indeed, recent media reporting has suggested that limited supply of Quillaja Saponaria bark extract drove a major pharmaceutical company to choose between developing a shingles vaccine for high-income markets or a TB vaccine for low-income countries (Reference Barry-Jester19). There is therefore a real risk of overexploitation of this resource for pharmaceutical applications, even as this tree faces greater risk of destruction from larger and more frequent bushfires due to climate change in Chile (Reference Villagra and Paula20). Viewed through the lens of incorporating environmental sustainability factors into HTA, Quillaja Saponaria therefore exemplifies multiple challenges, including risks of accelerating biodiversity loss through overexploitation; value choices over the allocation of scarce natural resources between low- or high-income and profit markets or products; and resilience risks from building dependency on an ingredient that itself may become increasingly endangered by the effects of climate change.
The initial operationalization of the concept of intrinsic versus generic environmental consequences might be achieved via a set of screening criteria for the presence of intrinsic risks. Such a screening process or tool would commence with identifying the key components (e.g. active ingredients, delivery agents/systems) and asking whether these components themselves are or contain:
-
i. greenhouse gases, chlorofluorocarbons and so on (e.g. pMDI asthma inhalers);
-
ii. sourced from threatened species or extracted from within at-risk ecosystems (e.g. Quillaja Saponaria);
-
iii. components, metabolites, or waste products and residues may have a prima facie high risk of ecotoxicity (e.g. diclofenac) (Reference Oaks, Gilbert and Virani21);
-
iv. sourced or manufactured exclusively in regions known to be at high risk of climate change impacts/disruption.
Additional screening criteria might also consider:
-
v. Whether this new product seeks to replace an existing intervention with a significant ecological impact?
-
vi. Is it reasonable to believe that this product might be employed at a very large scale (numbers of potential users × duration of use, e.g. glucagon-like peptide-1 analogs, statins)?
Optimizing the contribution of HTA to healthcare sustainability
The salience of healthcare environmental sustainability in general, and of the need to move to low-carbon and resilient healthcare systems in particular, has risen rapidly in both prominence and urgency as policymakers have been forced by events to acknowledge the severity of the ecological and climate crisis. Yet healthcare systems and HTA agencies have only limited access to a still tiny pool of people equipped with the necessary analytical skills to undertake life cycle assessments and other sustainability analyses. It is tempting to draw parallels with the very early days of HTA itself: the establishment of HTA hurdles for product adoption drove industry demand for health economics skills, and over time this demand led to increased training capacity and a large growth in health economist numbers. Yet there are two reasons why relying on a similar organic, market-driven approach is not viable as a strategy for responding to the need to incorporate environmental impacts within HTA. One is sheer urgency: this work must start immediately and build very quickly – this requires direct and deliberate prioritization decisions from the outset, which cannot be handed off to industry and academia. The second is the vital importance of ensuring that HTA approaches to environmental and climate sustainability are explicitly and closely nested within the broader web of healthcare sustainability policy mechanisms and levers described earlier in this paper. Allowing environmental HTA to “go it alone” and develop organically outside broader healthcare sustainability strategy is one sure way to undermine and hobble its ultimate effectiveness in this vital policy space. HTA is a necessary but absolutely not sufficient component for sustainable health technologies.
HTA agencies and practitioners must therefore take their place within overall national and international efforts to develop and execute robust and effective system-wide sustainability policy and strategy for healthcare. Prioritization of scarce skills and resources within this field will be essential; indeed there is a very strong argument for a concerted international effort to pool these limited resources to develop global solutions across all income levels and to avoid wasteful duplication of effort at national level. This is true both for HTA and for the wider policy and regulatory frameworks needed for action on generic impacts. The differentiation between intrinsic and generic environmental consequences and characteristics proposed in this Perspective provides one approach to assist with this prioritization of effort, but requires the development of simple and robust screening tools to allow rapid assessment of which category new products fall into. Meanwhile, the need to minimize environmental impacts provides yet another reason for HTA to lift its game in de-implementing low-value technologies in the existing technology stock – long called for, yet with limited success to date. Finally, the growing importance of resilience in healthcare systems will require different, more qualitative ways of thinking within HTA – assessing supply chain risks and vulnerabilities to discontinuities, rather than the simple application of cost-effectiveness thresholds.
Funding statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interest
The author declares none.