Environmental contamination plays an important role in the transmission of several key healthcare-associated pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), Clostridioides difficile, and Acinetobacter. Ample evidence supporting the role of the contaminated surface environment in the transmission of healthcare pathogens has been published: surfaces are frequently contaminated; pathogens survive for days (eg, vegetative bacteria) to months (ie, C. difficile spores); contact with surfaces results in hand and/or glove contamination; disinfection reduces surface and hand contamination via touch and healthcare-associated infections; rooms are inadequately cleaned and disinfected; patients admitted to a room previously occupied by a patient colonized or infected with a pathogen (eg, MRSA, VRE, or C. difficile) have an increased likelihood of developing colonization or infection with that pathogen, which can be reduced by improved terminal disinfection (eg, ultraviolet irradiation [UV]).Reference Weber, Anderson and Rutala1,Reference Rutala and Weber2 However, a limitation of “no touch” technologies, such as UV, is that they can only be used for terminal room disinfection because they require removal of all persons from the room. In addition, microbial contamination of environmental surfaces and noncritical patient-care items occurs continuously via patients, visitors, and staff. The intent of routine (eg, daily) disinfection is to make surfaces and equipment hygienically clean (not sterile), that is, free of pathogens in sufficient numbers to prevent human disease.Reference Rutala and Weber2,Reference Huslage, Rutala, Sickbert-Bennett and Weber3 If an antimicrobial residue remains on a disinfected surface and it persists on the surface for 24 hours, it could guard against recontamination with healthcare pathogens for 24 hours. In this study, we evaluated a novel disinfectant that is registered by the Environmental Protection Agency (EPA) to kill microbes on surfaces for at least 24 hours.
Methods
We investigated the continuously active disinfectant (CAD) against healthcare pathogens using an EPA “Protocol for Residual Self-Sanitizing Activity of Dried Chemical Residuals on Hard, Non-Porous Surfaces.”4 The method simulates contact and touches by incorporating “wear” of the test surface as well as reinoculations of the test and control surfaces over 24 hours. The test surfaces were inoculated with 10Reference Schmidt, Fairey and Attaway5 test organisms (9 test microbes, see Table 1), treated with the novel disinfectant, allowed to dry, and then abraded using a standardized abrasion machine under multiple alternating wet and dry wiping conditions (6 dry cycles, 6 wet cycles, total 12 cycles [2 passes per cycle = 24 passes]) interspersed with 6 reinoculations with 10Reference Huslage, Rutala, Sickbert-Bennett and Weber3 colony-forming units (CFU) of the test pathogen. The protocol requires a Gardco Washability and Wear tester (Gardner, Pompano Beach, FL) to perform the repeat abrasion portion of the test. After 24 hours, the surface was reinoculated (10Reference Hardy, Gossain and Henderson6 CFU) a final time, and the ability of the disinfectant to kill >99.9% of the 9 test microbes within 5 minutes was measured on 3 test surfaces: glass, formica, and stainless steel. The neutralizer used in the test was 1.5% lecithin and 5% Tween 80 (w/v) in sterile distilled water.
Table 1. Log10 Reduction of a Novel Disinfectant with Persistent Antimicrobial Activity
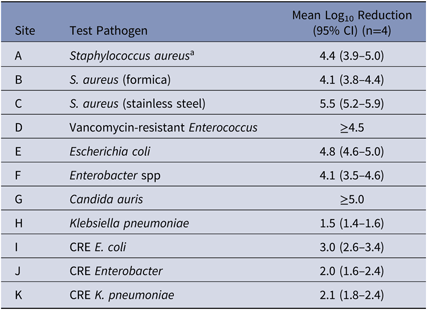
Note. CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae.
a Test surface was glass.
The novel disinfectant is EPA registered as Firebird F130 (Microban Products, Huntersville, NC) and marketed by Professional Disposables International (Woodcliff Lake, NJ). It has a 24-hour residual disinfectant claim and contains (w/w): 0.276% alkyl dimethyl benzyl ammonium chloride (50%C14, 40%C12, 10%C16); 0.104% didecyl dimethyl ammonium chloride; 0.207% octyl decyl dimethyl ammonium chloride; 0.104% dioctyl dimethyl ammonium chloride; 68.61% ethanol, as well as proprietary agents designed to increase longevity on the surface.Reference Schmidt, Fairey and Attaway5
Results
The novel disinfectant demonstrated a 3–5 log10 reduction in 5 minutes when testing S. aureus, VRE, C. auris, carbapenem-resistant E. coli and antibiotic-sensitive strains of E. coli, and Enterobacter spp (Table 1). The disinfectant demonstrated lower reductions of carbapenem-resistant isolates of Enterobacter spp and K. pneumoniae, and of antibiotic-sensitive K. pneumoniae (∼2 log10 reduction in 5 minutes). When the novel disinfectant was compared to 3 other commonly used disinfectants using the same methodology with S. aureus, the mean log10 reductions were as follows: 4.4 for novel disinfectant; 0.9 for quaternary ammonium compound with alcohol; 0.2 for improved hydrogen peroxide; and 0.1 for chlorine.
Discussion
After cleaning and disinfection, surfaces can rapidly become recontaminated.Reference Hardy, Gossain and Henderson6 Thus, hands or gloves of healthcare providers can become colonized or contaminated by touching contaminated environmental surfaces and patient-care equipment. Then, via inadequate hand hygiene or inappropriate glove use, healthcare providers can transfer healthcare pathogens to patients. Because routine cleaning of room surfaces by environmental services staff is frequently inadequate,Reference Rutala and Weber2 continuous room decontamination methods would fulfill an unmet need for cleaning and disinfection. The intent of this technology is to make surfaces hygienically clean (not sterile), that is, free of pathogens in sufficient numbers to prevent human disease. Technologies that could achieve and maintain low levels of microbial contamination include visible light disinfection (eg, high-intensity narrow-spectrum light), low concentration hydrogen peroxide, CAD, and self-disinfection surfaces (eg, copper).Reference Rutala and Weber2 These technologies highlight the potential to interrupt transmission from contaminated surfaces and equipmentReference Suwantarat, Supple, Cadnum, Sankar and Donskey7 by pathogen elimination from surfaces via healthcare provider hands and suboptimal compliance with hand hygiene or inappropriate glove use. These technologies are under active investigation to evaluate clinical efficacy, but to date, only copper has been assessed for the ability to reduce HAIs.
Our findings, which demonstrate the 3–5 log10 reductions of epidemiologically important pathogens in 5 minutes over 24 hours using a new CAD, are promising. The reason for a ∼2-log10 reduction (99% reduction) with carbapenem-resistant Enterobacter and Klebsiella and sensitive Klebsiella pneumoniae in our study is unclear. Another investigator found a 4 log10 reduction for these pathogens (personal communication, C Donskey), and most surfaces have <100 CFU/Rodac (25 cm2) in the clinical environment.Reference Huslage, Rutala, Sickbert-Bennett and Weber3 Importantly, the novel disinfectant achieved significant sustained antimicrobial activity in 5 minutes against most pathogens after 24 hours of “wear” and reinoculations. The results of this comparative evaluation demonstrate both no residual efficacy for the chlorine or improved hydrogen peroxide and nonsubstantial residual antimicrobial efficacy for the quaternary ammonium compound.
If the microbial load on surfaces is pathogen free or if pathogens are substantially reduced, the treated surface will not act as reservoir for pathogens and, thus, will not be linked to disease transmission. Thus, CADReference Schmidt, Fairey and Attaway5,Reference Tamimi, Carlino and Gerba8,Reference Rutala and Weber9 (and other continuous room decontamination technologies) may reduce or eliminate the problem of recontamination and minimize the role of contaminated environmental surfaces and equipment in transmission of healthcare pathogens.
These data are preliminary, and further studies are needed to determine whether the use of this disinfectant in a clinical environment reduces both microbial contamination and, ultimately, healthcare-associated infections. This study also has several potential limitations. Only 3 surfaces (ie, glass, formica, and stainless steel) were tested, and some surfaces (eg, bed rails) may undergo more touches than the number used for testing in this study. A pilot study with this CAD demonstrated superior reduction of microbial load over 24 hours compared to a dilutable quaternary ammonium compound or a disinfectant with ethanol and a quaternary ammonium.Reference Schmidt, Fairey and Attaway5 Other considerations include frequency of cleaning, effect of other disinfectants used on healthcare surfaces, and possible development of resistance by microbes to the CAD chemistry. The latter issue has been reviewed, and to date, no evidence has shown that using recommended antiseptics and disinfectants for >40 years selects for germicide-resistant or antibiotic-resistant organisms.Reference Weber and Rutala10 In regard to the frequency of use, we argue that the use of a CAD should not alter the frequency of cleaning and disinfection because one purpose of routine cleaning and disinfection is to remove dirt and debris in addition to reducing microbial contamination. The CAD can be removed from the surface by chlorine, accelerated hydrogen peroxide, and a detergent. A limitation of this technology is that it requires the application of the product to the surface to work, so thoroughness of application is essential.
Continued research and evaluation of the clinical value of continuous room decontamination (including CAD) are warranted as a means of reducing or eliminating environmental contamination in the transmission of healthcare-associated pathogens and decrease healthcare-associated infections.
Acknowledgements
The Gardco tester (Gardner, Pompano Beach, FL) was loaned to UNC Hospitals by Professional Disposables International. The novel disinfectant (Firebird F130) was provided by Microban Products (Huntersville, NC).
Financial support
This study was supported by UNC Health Care and the Centers for Disease Control and Prevention’s Prevention Epicenters Program (grant no. U54CK000483).
Conflict of interest
Drs Rutala and Weber are consultants for Professional Disposable International. All other authors report no conflict of interest relevant to this article.



