Introduction
With its high morbidity and lack of effective treatments, cognitive deficits in the spectrum of dementia are a devastating public health problem around the world, especially in ageing societies (Anderson, Reference Anderson2019; Oh and Rabins, Reference Oh and Rabins2019). Thus, identifying any modifiable factors promoting cognitive health could have a great impact on public health. Despite intensive research in the past few decades, experimental interventions targeting the main kinds of dementia have failed and no alternate pharmacological interventions have arisen in recent years (Lanni et al., Reference Lanni, Fagiani, Racchi, Preda, Pascale, Grilli, Allegri and Govoni2019), such that lifestyle interventions are important viable approach of proven efficacy for delaying the progression from mild cognitive impairment (MCI) to dementia (Kivipelto et al., Reference Kivipelto, Mangialasche and Ngandu2018).
Excessive alcohol consumption is indisputably a risk factor for cardiovascular disease (Nilssen et al., Reference Nilssen, Averina, Brenn, Brox, Kalinin and Archipovski2005), and some earlier reports of cardioprotective effects of low alcohol consumption have been called into question (Cífková and Krajčoviechová, Reference Cífková and Krajčoviechová2019). Coffee and tea consumption has been considered less problematic, and moderate consumption is linked to health risk reduction. Given the pharmacological effects of alcohol and caffeine on brain function, the association of their consumption with preservation of cognitive functioning is of great interest. Health benefits from tea (especially green tea) may be attributable to anti-inflammatory polyphenolic compounds (Poole et al., Reference Poole, Kennedy, Roderick, Fallowfield, Hayes and Parkes2017). However, results have been discordant, with some reports of protection from dementia among alcohol/coffee users (Sugiyama et al., Reference Sugiyama, Tomata, Kaiho, Honkura, Sugawara and Tsuji2016), and others indicating an increased risk of cognitive decline and dementia (Grønkjær et al., Reference Grønkjær, Flensborg-Madsen, Osler, Sørensen, Becker and Mortensen2019; Silva-Peña et al., Reference Silva-Peña, García-Marchena, Alén, Araos, Rivera, Vargas, García-Fernández, Martín-Velasco, Villanúa, Castilla-Ortega, Santín, Pavón, Serrano, Rubio, Rodríguez de Fonseca and Suárez2019). Some evidence shows that tea intake might decrease the risk for dementias and improve cognitive ability in healthy ageing (Mancini et al., Reference Mancini, Beglinger, Drewe, Zanchi, Lang and Borgwardt2017), whereas other research shows that consumption specifically of black tea (but not green tea) has cognitive protective effects (Shen et al., Reference Shen, Xiao, Ying, Li, Zhai, Shang, Li, Wang, He and Lin2015).
Given these varying results, it would be important to establish clear evidence-based recommendations about the risks and benefits of alcohol, coffee and tea intake for cognitive function in ageing populations. We, therefore, conducted a dose–response meta-analysis of published studies in normal elderly populations on the associations between alcohol, coffee and tea consumption and the risk of MCI or dementia.
Methods
We performed this meta-analysis following the procedures outlined by the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group (Stroup et al., Reference Stroup, Berlin, Morton, Olkin, Williamson, Rennie, Moher, Becker, Sipe and Thacker2000), and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Shamseer et al., Reference Shamseer, Moher, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015). The protocol was registered in PROSPERO (CRD42020175580).
Literature search
Four databases [the Medline (searched by PubMed), the Web of Science, the Embase and the Cochrane] were searched up to 4th June 2020. Our key words included ‘alcohol’, ‘ethanol’, ‘drink’, ‘wine’, ‘coffee’, ‘caffeine’, ‘coffea’, ‘chicory’, ‘tea’, ‘Alzheimer’, ‘AD’, ‘dementia’, ‘cognition’, ‘cognitive’ and ‘MCI’. The details of our searching strategy in each database are shown in online Supplementary material. We also searched the reference lists in other relevant meta-analysis to obtain the most complete compilation possible of the published literature. Moreover, if several publications came from the same study, we only included the study with the longest follow-up time and/or largest sample size. The articles were searched and managed using Endnote 9.0 software.
Selection criteria and data extraction
Our including criteria were as follows: (1) prospective cohort studies or nested case-control studies investigating the risk factors of cognitive deficits (defined as all types of dementia or MCI; the diagnostic criteria for cognitive deficits in each included study are shown in online Supplementary Table 1); (2) studies providing the dose-specific adjusted relative risks (RRs), hazard ratios (HRs) or odds ratios (ORs) with their 95% confidence intervals (CIs) for the association between drinking alcohol/coffee/tea and prospective development of cognitive deficits. However, we excluded studies according to the following criteria: (1) not published in English; (2) not of prospective cohort design or without specification of the follow-up time; (3) without the RRs/HRs/ORs of alcohol/coffee/tea drinking for cognitive deficits and their 95% CIs, or if these endpoints could not be calculated from the published data; (4) reported on cognitive decline rather than cognitive deficits per se; (5) included subjects with cognitive deficits at baseline who were not excluded in the calculation of RRs/HRs/ORs; (6) had follow-up time <3 years or follow-up rate <60% and (7) compared drinking with not-drinking, without presenting the doses. For details, see Fig. 1 and online Supplementary Table 2.
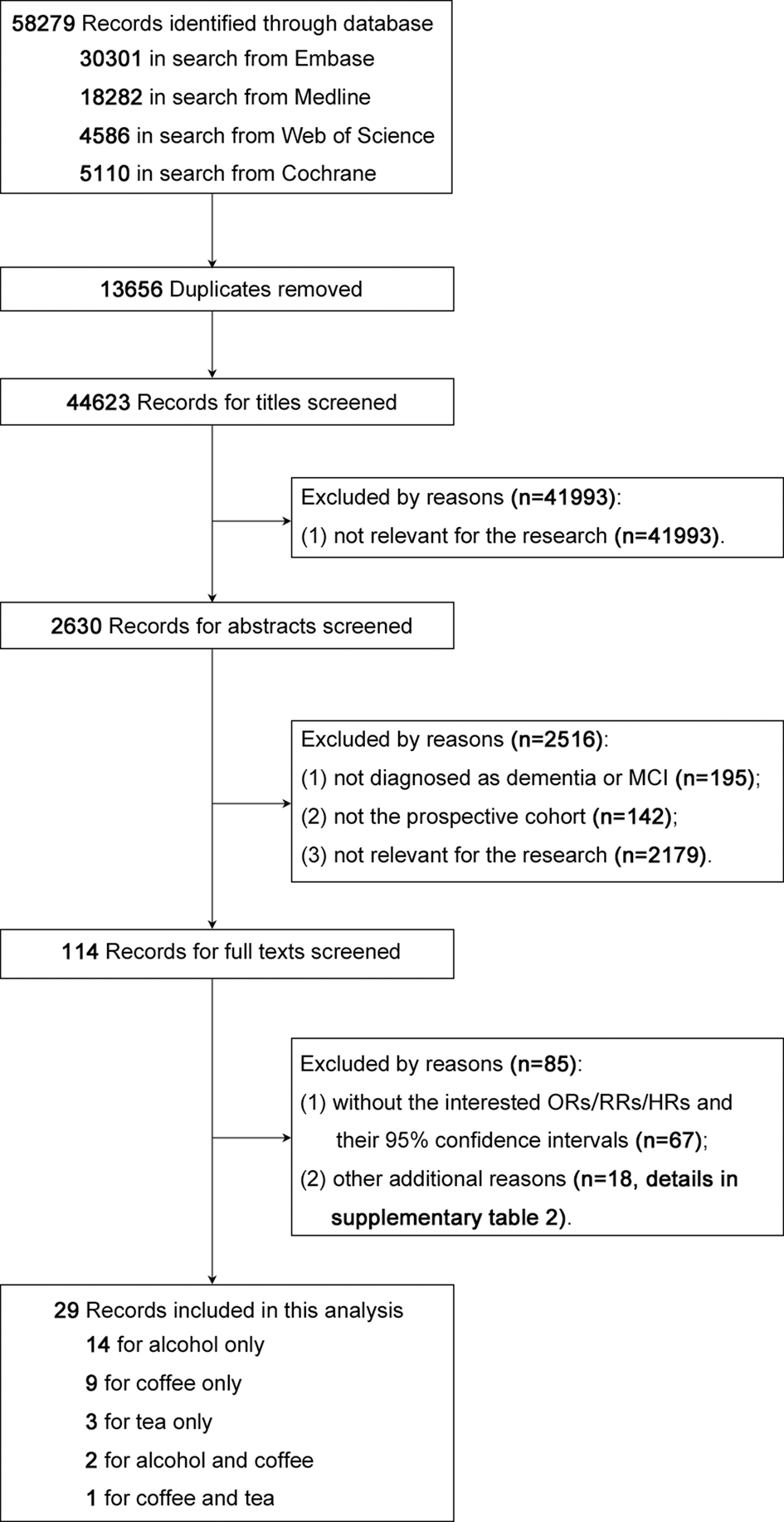
Fig. 1. Flow diagram of the literature search.
The following information was extracted from each included publication: first author name, publication year, diagnostic criteria, sample size, average age at baseline, the proportion of males, average follow-up time, daily doses of consumption of alcohol, coffee or tea, adjusted RRs /HRs/ORs and their 95% CIs, the number of participants and cases for each exposure level and the main covariates of MCI, Alzheimer's disease (AD) or any dementia. Two authors (Ran and Liu) searched the databases and extracted the data independently. Disagreements were resolved by consensus following discussion. If we were doubtful about the interpretation of a study, or some important data were not at study text, we contacted the corresponding author via email.
Quality assessment
The quality of the studies was assessed with the Newcastle-Ottawa scale (NOS), which ranges from 0 to 9 (best quality), as indicated in online Supplementary Table 3.
Statistical analysis
The primary outcome was cognitive deficits, which we defined in the broadest sense to include MCI, AD or any dementia. If a study reported the RRs of MCI or dementias and a composite risk of MCI and dementia taken together, we chose the composite results. The secondary outcome was dementia of all types (excluding MCI). Diagnosis of MCI and dementia was based on various criteria, i.e. DSM (Diagnostic and Statistical Manual of Mental Disorders) and ICD (International Statistical Classification of Diseases and Related Health Problems). In the analysis of tea consumption, we only focused on the results of green tea, because of the limited number of studies on other types of tea.
To take advantage of the high-quality research studies and obtained the stable and reliable results, we converted these studies to standard drink units as required. For the case of alcohol, consumption was converted to g/day, where one drink equivalent equalled 12 g in the United States, 21.2 g in Japan and 10 g in Australia and Europe (Reynolds et al., Reference Reynolds, Lewis, Nolen, Kinney, Sathya and He2003), and 2.4 g/day equalled consumption of one drink equivalent per week (Xu et al., Reference Xu, Wang, Wan, Tan, Li, Tan and Yu2017). One drink equivalent corresponded to a standard unit, i.e. one glass of wine (Virtanen et al., Reference Virtanen, Jokela, Nyberg, Madsen, Lallukka, Ahola, Alfredsson, Batty, Bjorner, Borritz, Burr, Casini, Clays, De Bacquer, Dragano, Erbel, Ferrie, Fransson, Hamer, Heikkilä, Jöckel, Kittel, Knutsson, Koskenvuo, Ladwig, Lunau, Nielsen, Nordin, Oksanen, Pejtersen, Pentti, Rugulies, Salo, Schupp, Siegrist, Singh-Manoux, Steptoe, Suominen, Theorell, Vahtera, Wagner, Westerholm, Westerlund and Kivimäki2015). Consumption frequencies were several times per month (>0.6 g/day), monthly drinking (0.6–2.4 g/day), weekly drinking (2.4–16.8 g/day) and daily drinking (>16.8 g/day). For coffee and tea drinking, rarely or never drinking was scored as 0, occasional drinking or 1–6 days/week corresponded to 0–1 cup/day and every day was score as ⩾1 cup/day. In studies of caffeine as the exposure factor, we converted it to units of caffeinated beverage scaled to a dose of 115 mg for one cup of coffee (Paganini-Hill et al., Reference Paganini-Hill, Kawas and Corrada2016). If the consumption rates were not the same for women and men, we chose the median. To obtain quantitative scores of exposure levels, we assigned the mean or median dose of each exposure level to a corresponding loge of the RR, if reported in the study. Otherwise, we designated the dose as the midpoint of upper and lower bounds of the range for a given level of consumption. If the drinking doses did not have a specified upper bound, we assigned the lower bounds of that level by a multiplicative factor of −1.25 (Brien et al., Reference Brien, Ronksley, Turner, Mukamal and Ghali2011).
We used the method described by Greenland and Longnecker to conduct the linear or nonlinear dose–response meta-analysis (Greenland and Longnecker, Reference Greenland and Longnecker1992; Orsini et al., Reference Orsini, Li, Wolk, Khudyakov and Spiegelman2012). The generalised least squares regression was used to estimate the relationship between the natural logarithm of the relative risks (RRs/HRs/ORs) and the exposure level or its transformation. We used the Hamling method to re-calculate the RRs and 95% CI if the reference level was not at the lowest level (Hamling et al., Reference Hamling, Lee, Weitkunat and Ambühl2008). For each outcome (primary and secondary) and exposure (alcohol, coffee and tea) we first used a cubic spline transformation with four knots based on Harrell's recommended percentiles of the distribution of exposure levels to estimate any nonlinear relationship. If the nonlinear trend was not confirmed even though the entire model was significant, we then fitted the linear models using the original exposure levels. A fixed or random effects model was chosen according to the heterogeneity between studies. We used the Q and I 2 statistics to test the data heterogeneity. The presence of data heterogeneity was confirmed when the p-value derived from the Q statistic was <0.05 or when the I 2 statistic was >50%. The presence of publication bias was assessed by funnel plots and Egger's test.
To avoid potential confounds due to age, we did a sub-group analysis for the primary and secondary outcomes according to the population's dichotomised average age at baseline (age <60 and age ⩾60 years). We conducted a sensitivity analysis for the primary outcome, and excluded studies reporting dementia-related death (as the outcome) or daily caffeine consumption (as the exposure).
The dose–response meta-analyses were conducted using Stata 15.0 software, and the glst and xblc commands were used to perform the model estimation and plot the linear or nonlinear dose–response relationship graphs.
Results
Figure 1 depicts the process of searching for and selecting the included studies. After removing replicates discovered in the four databases, there remained 44 623 studies, of which the great majority (n = 41 993; 94%) were deemed irrelevant based on their titles. Of the 2630 remaining studies, we excluded 195 after reading the abstracts due to the lack of specification of dementia or MCI, 142 of which were not prospective cohort studies, and 2179 were not relevant to the topic. Next, 114 studies were assessed in detail, of which 67 were excluded due to the lack of RRs/HRs/ORs and their 95% CIs, and 18 studies were excluded for other reasons (details in online Supplementary Table 2). Finally, 29 studies were included in our meta-analysis.
Description of the studies included
Of the 29 included studies, 14 (48%) were about alcohol consumption only (Huang et al., Reference Huang, Qiu, Winblad and Fratiglioni2002; Ruitenberg et al., Reference Ruitenberg, van Swieten, Witteman, Mehta, van Duijn, Hofman and Breteler2002; Truelsen et al., Reference Truelsen, Thudium and Grønbaek2002; Mukamal et al., Reference Mukamal, Kuller, Fitzpatrick, Longstreth, Mittleman and Siscovick2003; Anttila et al., Reference Anttila, Helkala, Viitanen, Kareholt, Fratiglioni, Winblad, Soininen, Tuomilehto, Nissinen and Kivipelto2004; Espeland et al., Reference Espeland, Gu, Masaki, Langer, Coker, Stefanick, Ockene and Rapp2005; Jarvenpaa et al., Reference Jarvenpaa, Rinne, Koskenvuo, Raiha and Kaprio2005; Bowen, Reference Bowen2012; Zhou et al., Reference Zhou, Zhou, Zhong, Li, Tan and Zhou2014; Handing et al., Reference Handing, Andel, Kadlecova, Gatz and Pedersen2015; Langballe et al., Reference Langballe, Ask, Holmen, Stordal, Saltvedt, Selbaek, Fikseaunet, Bergh, Nafstad and Tambs2015; Heffernan et al., Reference Heffernan, Mather, Xu, Assareh, Kochan, Reppermund, Draper, Trollor, Sachdev and Brodaty2016; Ormstad et al., Reference Ormstad, Rosness, Bergem, Bjertness, Strand and Grp2016; Sabia et al., Reference Sabia, Fayosse, Dumurgier, Dugravot, Akbaraly, Britton, Kivimaki and Singh-Manoux2018), nine (31%) about coffee consumption only (Eskelinen et al., Reference Eskelinen, Ngandu, Tuomilehto, Soininen and Kivipelto2009; Laitala et al., Reference Laitala, Kaprio, Koskenvuo, Raiha, Rinne and Silventoinen2009; Gelber et al., Reference Gelber, Petrovitch, Masaki, Ross and White2011; Mirza et al., Reference Mirza, Tiemeier, de Bruijn, Hofman, Franco, Kiefte-de Jong, Koudstaal and Ikram2014; Loftfield et al., Reference Loftfield, Freedman, Graubard, Guertin, Black, Huang, Shebl, Mayne and Sinha2015; Solfrizzi et al., Reference Solfrizzi, Panza, Imbimbo, D'Introno, Galluzzo, Gandin, Misciagna, Guerra, Osella, Baldereschi, Di Carlo, Inzitari, Seripa, Pilotto, Sabba, Logroscino and Scafato2015; Driscoll et al., Reference Driscoll, Shumaker, Snively, Margolis, Manson, Vitolins, Rossom and Espeland2016; Sugiyama et al., Reference Sugiyama, Tomata, Kaiho, Honkura, Sugawara and Tsuji2016; Park et al., Reference Park, Freedman, Haiman, Le Marchand, Wilkens and Setiawan2017) and three (10%) about tea consumption only (Dai et al., Reference Dai, Borenstein, Wu, Jackson and Larson2006; Tomata et al., Reference Tomata, Sugiyama, Kaiho, Honkura, Watanabe, Zhang, Sugawara and Tsuji2016; Pan et al., Reference Pan, An, Liu, Khan, Yan and Wang2019). Two studies were about alcohol and coffee consumption (Paganini-Hill et al., Reference Paganini-Hill, Kawas and Corrada2016; Larsson and Wolk, Reference Larsson and Wolk2018), and one was about coffee and tea consumption (Noguchi-Shinohara et al., Reference Noguchi-Shinohara, Yuki, Dohmoto, Ikeda, Samuraki, Iwasa, Yokogawa, Asai, Komai, Nakamura and Yamada2014). In the studies of alcohol, one was about dementia-related mortality (Ormstad et al., Reference Ormstad, Rosness, Bergem, Bjertness, Strand and Grp2016) and two studies were case-control studies nested in a cohort (Truelsen et al., Reference Truelsen, Thudium and Grønbaek2002; Mukamal et al., Reference Mukamal, Kuller, Fitzpatrick, Longstreth, Mittleman and Siscovick2003). In the studies of coffee, two were about dementia-related mortality (Loftfield et al., Reference Loftfield, Freedman, Graubard, Guertin, Black, Huang, Shebl, Mayne and Sinha2015; Park et al., Reference Park, Freedman, Haiman, Le Marchand, Wilkens and Setiawan2017), and two studies united several types of drinking to calculate net caffeine consumption (Driscoll et al., Reference Driscoll, Shumaker, Snively, Margolis, Manson, Vitolins, Rossom and Espeland2016; Paganini-Hill et al., Reference Paganini-Hill, Kawas and Corrada2016). As shown in online Supplementary Table 1, the publication dates ranged from 2002 to 2019. Of the 29 studies, nine (31%) were conducted in the USA, of which two were about Japanese Americans. The most common diagnostic criteria were DSM and ICD. Most of the studies (27; 93%) concerned dementia, three reported on MCI, and four pooled various cognitive deficits. In each case, the NOS score almost all exceeded 8 (online Supplementary Table 3).
Alcohol drinking and cognitive deficits
The results from 16 studies showed a nonlinear relationship between alcohol drinking and risk of cognitive deficits (p for non-linearity < 0.05). Compared with non-drinkers, the incidence of cognitive deficits was lower with low consumption (<11 g/day), but the effect of heavier drinking (>11 g/day) was not significant (Fig. 2a). A similar dose–response relationship was observed with outcome of dementia, excluding MCI (Fig. 2b), with a lower risk observed for <11 g/day alcohol. We also conducted a subgroup analysis of the relationship between alcohol drinking and cognitive deficits according to the populations’ average age at baseline. In the 11 trails of average age ⩾60 years, consumption of <17 g/day alcohol imparted a lower risk of cognitive deficits according to the nonlinear plot (Fig. 2c). The dose–response relationship of the five studies with populations <60 years old also showed an association between low alcohol consumption and cognitive health, but the cut-off value threshold was <7.5 g/day (Fig. 2d). In the sensitivity analysis, after excluding the study of Ormstad et al. about dementia-related death (Ormstad et al., Reference Ormstad, Rosness, Bergem, Bjertness, Strand and Grp2016), a similar nonlinear relationship was found whereby mild drinking (<11 g/day) was a protective factor (online Supplementary Fig. 1).

Fig. 2. Dose–response meta-analyses for dementias or MCI (a), dementia (b) and alcohol intake. Mild alcohol consumption (<11 g/day) is associated with a reduced risk compared with non-drinking. Subgroup meta-analyses for the population aged ⩾60 years (c) and <60 years (d). The corresponding cut-off values for protection of risk for dementia or MCI were 17 g/day (c) and 7.5 g/day (d). MCI = mild cognitive impairment.
Coffee drinking and cognitive deficits
Figure 3 depicts the nonlinear relationship between coffee consumption and cognitive deficits in 12 studies (p for non-linearity < 0.05). A dose-dependent relationship was observed, whereby mild coffee drinkers (< 2.8 cups/day) had a lower risk of cognitive deficits than non-drinkers (Fig. 3a). Similarly, mild coffee drinking (<2.3 cups/day) was a protective factor for dementia with exclusion of MCI (Fig. 3b). With increasing coffee consumption, the relationship with cognitive outcome became non-significant. Subgroup meta-analysis was also conducted for the (n = 8) studies of average age ⩾60 years at baseline. Here, the nonlinear relationship indicated that coffee consumption <4 cups/day imparted a lower risk of cognitive deficits compared with non-drinking (Fig. 3c). However, coffee drinking was not a significant protective factor for cognitive deficits in groups of average age <60 years (Fig. 3d).

Fig. 3. Dose–response meta-analyses for dementia or MCI (a), dementia (b) and coffee intake. Mild coffee consumption [2.8 cups/day (a), 2.3 cups/day (b)] is associated with a reduced risk of cognitive deficits compared with non-drinking. Subgroup meta-analyses for the population over aged ⩾60 years (c), and the cut-off values for protection against dementia or MCI come to less than 4 cups/day. Subgroup meta-analyses for the population aged <60 years (d), and no dose of coffee drinking was significantly protective against dementia or MCI. MCI = mild cognitive impairment.
The studies of Park et al. and Loftfield et al. were about dementia-related death in relation to coffee consumption (Loftfield et al., Reference Loftfield, Freedman, Graubard, Guertin, Black, Huang, Shebl, Mayne and Sinha2015; Park et al., Reference Park, Freedman, Haiman, Le Marchand, Wilkens and Setiawan2017). The studies of Paganini-Hill et al. and Driscoll et al. chose caffeine as the exposure factor (Driscoll et al., Reference Driscoll, Shumaker, Snively, Margolis, Manson, Vitolins, Rossom and Espeland2016; Paganini-Hill et al., Reference Paganini-Hill, Kawas and Corrada2016). Upon removing these studies from consideration, there remained a significant nonlinear relationship such that mild coffee consumption was linked to a reduced risk for cognitive deficits (online Supplementary Figs 2–4).
Tea drinking and cognitive deficits
The data for tea drinking showed a linear relationship with morbidity from cognitive deficits (p for linearity < 0.05) (Fig. 4). Thus, tea consumption was a protective factor for cognitive health (RR, 0.94; 95% CI, 0.92–0.97); the linear relationship indicated that drinking one cup of tea per day brings a 6% reduction in cognitive deficits morbidity, whereas two cups per day brought an 11% decrease.

Fig. 4. Dose–response meta-analyses of the risk for dementia or MCI as a function of tea intake. The risk declined significantly with increasing tea consumption (RR, 0.94; 95% CI, 0.92–0.97). MCI, mild cognitive impairment; RR, risk ratio; 95% CI, 95% confidence interval.
Discussion
This meta-analysis included 29 studies presenting a dose–response investigation of the relationships between alcohol, coffee and tea drinking and cognitive health. We based this study on previous meta-analyses of the relationships between beverage consumption and risk of cognitive impairment, but made all efforts to cast the broadest possible net in support of a more systematic analysis, while maintaining strict inclusion criteria. We included very large samples (131 777 for alcohol, 333 843 for coffee and 20 411 for tea). Our study indicated that light consumption of alcohol (<11 g/day) and coffee (<2.8 cups/day) might be independently associated with reduced risk for developing cognitive deficits compared to abstinence. The cognitive benefits of tea consumption seemingly increased with daily dosage.
In consideration of the association with alcohol consumption, the cut-off value for risk of dementia was the same as for the composite of dementia and MCI, which suggests a possible common protective pathway, irrespective of diagnosis. Light coffee consumption (<2.8 cups/day) had similar associations with cognitive health as those seen for light alcohol consumption. We based this coffee cut-off for the risk for MCI or dementia relative to that of non-drinkers. As dementia diagnosis qualifies as a severe cognitive deficit, the criterion for its prevention must be stricter than for MCI. Insofar as caffeine is plausibly the main neurophysiologically active constituent of coffee, we can tentatively attribute the precognitive effects of caffeine to purinergic receptor antagonism. Our results showing a linear relationship between cognitive benefits with increasing tea consumption lead us to consider that tea may be the preferred beverage for cognitive protection, also given the lack of any evidence for detrimental effects of tea drinking. In the current study, we focused on green tea, since there was insufficient meta-analytical data to support an analysis of pro-cognitive effects of black tea.
The meta-analysis study of Xu et al. discussed the dose–response relationship between alcohol consumption and dementia (Xu et al., Reference Xu, Wang, Wan, Tan, Li, Tan and Yu2017). In their compilation of 15 cohort studies, it was apparent that modest alcohol consumption (<12.5 g/day) could reduce the risk of dementias. However, since they did not consider MCI as an intermediate state likely to progress to dementia, our data may therefore constitute an update of the prior state of knowledge. Their dose–response analyses were separated by the various units (drinks/week, g/day and times/week) with a limited number of studies for each unit, which naturally results in selection bias and relatively low stability of the meta-analytic results. Two dose–response meta-analyses examined coffee drinking and cognitive impairment, but both had excluded studies of caffeine dose as distinct from the number of cups per day (Wu et al., Reference Wu, Sun and He2017; Larsson and Orsini, Reference Larsson and Orsini2018). The study of Larsson et al. did not show a significant pro-cognitive effect of coffee drinking, which may reflect their lack of comprehensive inclusion of relevant studies (Larsson and Orsini, Reference Larsson and Orsini2018). They also included two studies on dementia-related death, without considering how this endpoint may have influenced results by sensitive analysis. The meta-analysis by Wu et al., which included nine studies, found that low coffee consumption was good for cognitive health (Wu et al., Reference Wu, Sun and He2017). However, there was insufficient detail in their dose–response analysis to support strong conclusions. Furthermore, they included one study that only compared coffee drinkers and non-drinkers, without considering doses (Lindsay et al., Reference Lindsay, Laurin, Verreault, Hebert, Helliwell, Hill and McDowell2002), which was therefore unsuitable for dose–response analysis. There had not hitherto been any quantitative meta-analyses about tea consumption and cognitive health.
This study is not only an update of the precious meta-analyses, but also presents an original synthesis of the available data. Alcohol, coffee and tea consumption is a widespread, if not universal aspect of daily life in many countries around the world, but previous epidemiological studies of this type considered the three beverage types separately. In considering alcohol, coffee and tea together, we seek a broader understanding of the effects of these beverages on cognitive health. Using quantitative analysis methods, we obtain separately the dose–response relationships of these three types of beverages as mitigators against age-related cognitive deficits. Our findings support tentative recommendations to protect cognitive health, insofar as consuming <11 g/day alcohol per day, or daily consumption of tea or 2.8 cups of coffee emerged as being distinctly protective. Our subgroup analysis suggested that the dose cut-off values for the cognitive benefits of alcohol/coffee differ between those aged <60 years and older individuals.
MCI is a prodromal syndrome often progressing to dementia; our analysis included MCI together with dementia of all types. Cognitive decline is a broad term without clear diagnostic criteria. Furthermore, many clinical articles do not use ORs/HRs/RRs as the final indicator, which excludes them from inclusion in our meta-analysis. We assessed the publication bias by applying a funnel plot and Egger's test (online Supplementary Figs 5–9). The studies of coffee consumption in relation to MCI or dementia had small-study effects or publication bias (displayed in the left bottom in the funnel plot and Egger's test p = 0.039), but the other tests were not significantly biased. The nonlinear relationship was stable using the trim and fill method in the case where publication bias was present.
We endeavoured in this meta-analysis to unify various studies by adopting standard units of beverage consumption, which is a common problem in studies of this type. In the case of alcohol, we could convert the reported units to common units of ‘g/day’. In the case of coffee drinking, most researchers reported ‘cups/day’ as their standard unit, where a cup is about 237 ml. To take advantage of the high-quality research and obtain the most stable and reliable results, we converted these studies to standard units as required.
Limitations
We note some limitations of the current meta-analysis. First, we base our findings on data of observational studies, and their adjusted factors for ORs/RRs/HRs would therefore naturally differ. Although we chose the ORs/RRs/HRs with the most adjusted factors, our evidence rank was not as strong as seen with meta-analysis of individual patient data. Second, in our results, heavier consumption of alcohol/coffee was not associated with improved cognitive health. That result might reflect limitations related to sample size, such that further clinical studies would be necessary to establish clearly the possible pro-cognitive effects of heavier consumption. Third, we did not consider ethnic and societal differences in the study populations. The coffee studies were mostly of American and European origin, and the (green) tea studies were mostly of Asian origin. More cross-cultural evidence shall thus be needed to generalise our results to the whole world. The incidence of cognitive deficits in the elderly is influenced by genetic factors, which are not reported or discussed in most of the included studies. We cannot determine the influence of sex because most of the included studies analysed males and females together, leaving only limited data reported by gender. Finally, because of the lack of randomised controlled trial studies in this field, all the research included were observing studies. Therefore, some degrees of selection bias and confounding bias may have been unavoidable in our meta-analysis.
Conclusions
Overall, we are encouraged by the findings that light consumption of alcohol and coffee may benefit cognitive health, with evidence-based dose thresholds for imparting these benefits. We propose that everyday habits of beverage consumption should be considered as significant modifiable factors for the promotion of cognitive health, despite caveats about the systemic effects of regular alcohol consumption based on analysis of observational data. Future research should focus on these and other lifestyle factors with a potential to moderate cognitive deficits in the elderly.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796020001183
Data
The data sets used and analysed in the current study are available from the corresponding author on a reasonable request.
Acknowledgements
The authors acknowledge Inglewood Biomedical Editing.
Author contributions
Wei Wang and Minghuan Wang were responsible for the study design. Lusen Ran, Shabei Xu and Yuanyuan Fang were responsible for the literature search and study selection. Wenhua Liu and Lusen Ran were responsible for the data extraction and quality assessment. Minghuan Wang, Wenhua Liu and Lusen Ran were responsible for the statistical analysis and paper drafting. Xiang Luo, Jia Li, Dengji Pan and Shabei Xu were responsible for critical revision of the paper.
Financial support
None.
Conflict of interest
None.
Ethical standards
This research did not involve human and/or animal experimentation.






