INTRODUCTION
Chickenpox (varicella) is caused by a varicella-zoster virus infection. It is one of the most common vaccine-preventable childhood diseases in all countries, in addition to developed countries, without a vaccination programme [Reference Banz1, Reference Seward2]. It is highly contagious, and considered an easily recognizable disease because of the presence of a characteristic and often pathognomonic macular-papular-vesicular rash [Reference Heininger and Seward3]. It has long been considered a benign, self-limiting infectious childhood disease [Reference Boelle and Hanslik4]. However, serious complications including secondary bacterial skin and soft-tissue infections, cerebellitis, encephalitis, pneumonia and coagulopathy can occur, especially in high-risk groups such as neonates, adolescents and immunocompromised people [Reference Boelle and Hanslik4–Reference Marin6]. In total, 40·7–83·3% of children hospitalized with chickenpox will develop complications [Reference Carapetis, Russell and Curtis7–Reference Mandelcwajg11], and a mortality rate of 2–4/100 000 infected persons has been reported [Reference Heininger and Seward3]. Contrary to the general perception as a benign disease, varicella in children is a worldwide public health issue [12, Reference Baldo13].
The risk of acquiring a chickenpox infection in the non-vaccinated population exceeds 95%, and most people are infected under the age of 20 years [Reference Banz1]. Without a universal childhood vaccination programme, varicella incidence is significantly high in children aged <10 years and the peak is between 3 and 6 years [14]. In 1998, the World Health Organization (WHO) recommended that routine childhood varicella vaccination should be considered in the countries where chickenpox had important public health impact, where the vaccine was affordable, and where sustained and high vaccination coverage was achievable [15]. Studies from various countries such as the USA, Germany, and Taiwan have shown that the implementation of universal childhood immunization with varicella vaccine resulted in a significant decline in chickenpox incidence, complications, and mortality rate; as well as significant cost-savings from a societal perspective [Reference Seward2, Reference Giammanco16–Reference Zhou23].
The epidemiology of varicella varies in different geographical regions, climatic belts, population densities, and degrees of socioeconomic development [Reference Fairley and Miller24–Reference Yawn, Yawn and Lydick26]. In contrast to countries with a temperate climate, varicella has been reported to have a delayed onset of disease in tropical countries, such as India and Singapore [Reference Lokeshwar27, Reference Ooi28]. In Hong Kong, a recent study by Chan et al. reported an average annual varicella notification rate and hospital attendance rate in children aged <18 years of 981/100 000 and 285/100 000 of the paediatric population, respectively [Reference Chan29]. More than 90% of the chickenpox notifications were aged <18 years, with 29·1% of notified paediatric cases of chickenpox receiving treatment attending public or private hospitals [Reference Chan29]. The complications rate in children hospitalized for varicella was 47%. Despite the fact that a safe and effective licensed vaccine has been available for many years, there is no universal vaccination against varicella for children in Hong Kong. When local parents would like to have their children vaccinated against varicella, private practice or family doctors are the only choice. A recent survey conducted by the Department of Health in 2009 reported a low chickenpox vaccine coverage in local-born children of only 32·4% [Reference Chan30]. Studies in other countries found that parental concerns about vaccine safety, efficacy and side-effects were some of the major barriers against having their children vaccinated [Reference Chan30–Reference Meine32]. However, little is known about the barriers against vaccination uptake in Hong Kong [Reference Taylor and Newman33]. With a tropical climate and potential risk of delayed onset of varicella in adolescents and adults who are more prone to develop severe disease, it is essential to achieve a high and sustained vaccine coverage to prevent the shift of varicella epidemiology and reduce disease burden and severe outcomes caused by varicella in Hong Kong.
The objective of this study was to investigate the parental-reported varicella vaccination rate in preschool children aged between 2 and 6 years in Hong Kong. Furthermore, parental barriers against varicella vaccination were explored.
METHODS
A cross-sectional kindergarten-based parent-administered questionnaire survey was conducted in Hong Kong during 2012. We used the standard formula for estimating sample size (equation 4·19 in [Reference Scheaffer, Mendenhall and Ott34]) and assumed a conservative prevalence of 0·5 and a margin of error of 2·5%; around 1537 subjects were required. A complete list of kindergartens was then obtained from the Education Bureau of Hong Kong (http://www.edb.gov.hk/tc/index.html). Since the number of students was around 100–150 per kindergarten, we aimed to recruit 15 kindergartens. As we expected the response rate from kindergarten would be <50%, we sent out invitations to the principals of 40 randomly selected kindergartens according to the four major geographical regions of Hong Kong (10 per region) at the first attempt.
A total of 12 kindergartens agreed to take part in the study and there were over 1500 students in the kindergartens. Next, parents of the students from the participating kindergartens were invited to participate in the study. Parents who did not understand Chinese or English were excluded.
All parents were required to complete a self-administered questionnaire, in Chinese or English, with questions covering the following areas: (i) demographic characteristics of the participants; (ii) socioeconomic status of their family; (iii) history of chickenpox and associated medical consultation/hospitalization of participants and family members; (iv) history of chickenpox vaccination of participants and family members; and (v) reasons for having or not having their children vaccinated with chickenpox vaccine on a 4-level Likert scale. The measure of each construct was developed based on concepts from the health belief model including statements concerning perceived risk and severity, personal experience of varicella and cues to action [Reference Dubos8].
The questionnaire was developed and checked with the content validity by experts. Face validity was checked with pilot testing of the questionnaire with 20 parents of kindergarten students. All returned questionnaires were checked for completeness; and those questionnaires with missing data with personal identifiers of participants were sent back to the respective kindergartens to obtain the missing data.
Varicella prevalence and varicella vaccine coverage were calculated as percentages with a 95% confidence interval. The vaccine coverage was calculated by first excluding those with varicella history in the vaccination coverage calculation since they would not be eligible for vaccination. These ineligible subjects would also be excluded in the analyses thereafter.
For data on vaccination uptake, frequencies and percentages were computed for each of the predictive variables and the corresponding odds ratio (OR), 95% confidence intervals (CI) and P value were also computed. Those variables with P < 0·1 in univariate analysis were included in the multiple logistic regression analysis, and stepwise procedure was used to select the final model.
Parental concerns regarding their children's vaccination were also enquired about, with the top three reasons being reported. All statistical analysis was performed using SPSS software version 16·0 (SPSS Inc., USA) and P < 0·05 was taken as statistically significant.
RESULTS
In total, 1538 questionnaires were distributed to all students aged 2–6 years in 12 participating kindergartens. A total of 1285 parents consented to participate and returned the completed questionnaires, achieving an overall response rate of 83·6%. Participants included 687 (53·5%) males and 598 (46·5%) females, with a male to female ratio of 1·15 which was consistent with the ratio of 1·13 from the Census and Statistics Department of Hong Kong in 2010 [35]. The mean age of the students was 4·4 years. Characteristics of the students are summarized in Table 1.
Table 1. Demographic and socioeconomic characteristics by vaccination status of the participants
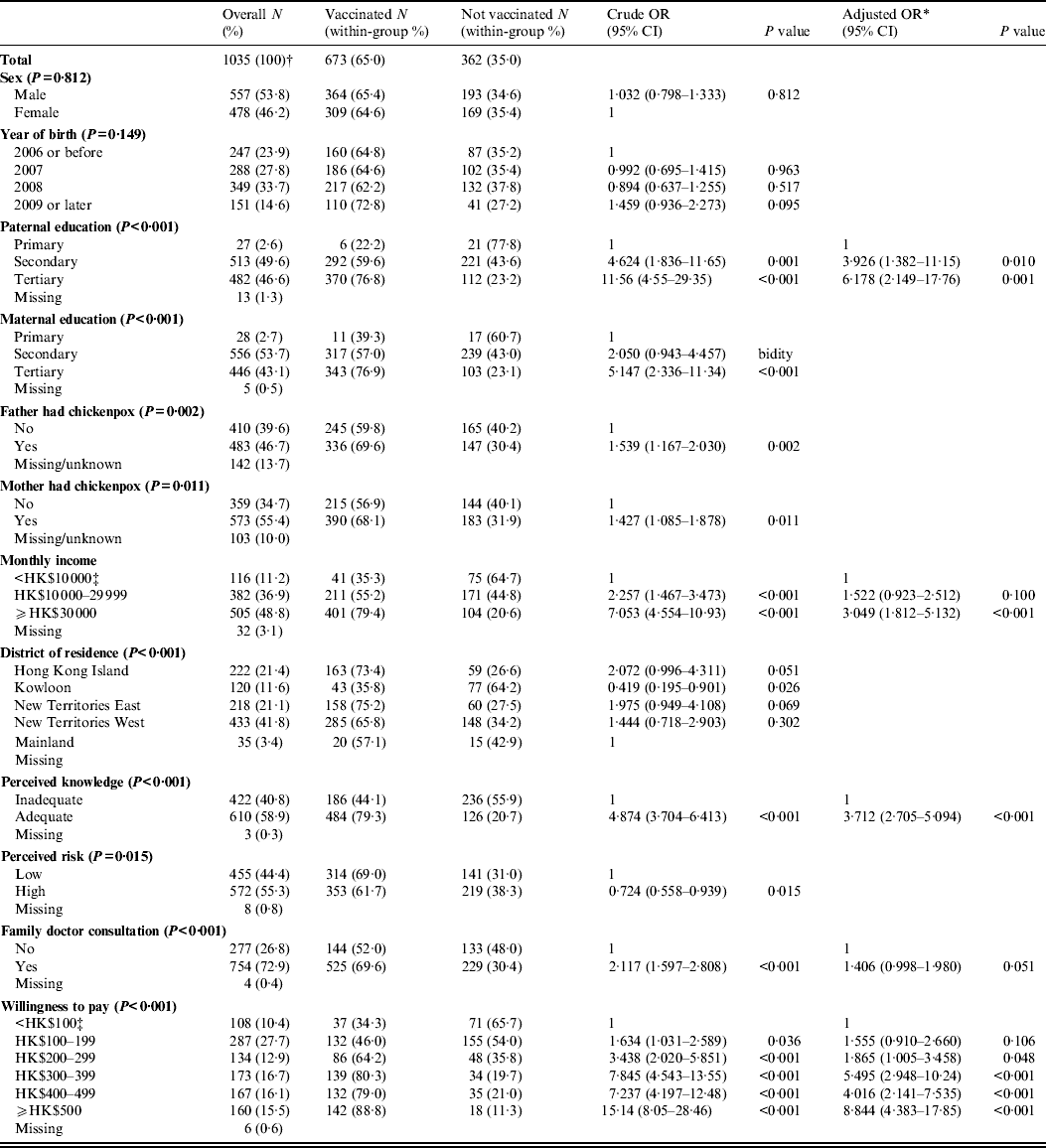
OR, Odds ratio; CI, confidence interval.
* The model was selected by stepwise procedure.
† 250 students, who had contracted chickenpox before, were excluded in the current analysis.
‡ US$1 = approx. HK$7.8.
Of the 1285 respondents, 250 (19·5%) parents reported that their children had previously contracted varicella, of which 93·2% had received medical care. In total, varicella vaccination coverage was 65·0% (673/1035). The actual varicella vaccination coverage ranged from 30·6% to 79·5% by kindergarten. In the univariate analyses, various potential predictive variables (including sex, year of birth, parents' education level, household income, etc.), on varicella vaccination status were explored and are summarized in Table 1. Significant associations for parental education, maternal education, parental previous varicella history, maternal previous varicella history, monthly household income, district of residence, perceived knowledge, perceived risk, family doctor consultation, and willingness to pay the consultation fees were found.
In the multivariate logistic regression analysis, only paternal education level, monthly household income, perceived knowledge, family doctor consultation and willingness to pay were found to be significantly associated with vaccine uptake status.
The top three factors for having their children vaccinated were recommendations by family doctors (96·6%), specialist doctors (94·1%), and healthcare professionals in school (91·1%), while the three major concerns for not having their children vaccinated were ‘unsure of the effect of vaccination’ (54·8%), ‘no recommendations from the Hong Kong Government’ (50·9%), and ‘no recommendation from the doctors’ (50·5%).
DISCUSSION
This is the first kindergarten-based study to investigate the varicella vaccination rate and parental barriers against having their children vaccinated with varicella vaccine in Hong Kong. Although the education levels of the participating parents were a slightly higher, our sample of participating parents was comparable with the data from the Census and Statistics Department and therefore considered representative of the general population in Hong Kong.
In the present study, the parent-reported varicella vaccination uptake rate was 65·0%, which was higher than the varicella vaccination rate of 32·4% found in a recent maternal and childcare centre-based survey in 2009 [Reference Chan30]. However, this is still far below the vaccination coverage of 82–89% in those countries with a universal varicella vaccination programme such as the USA, Australia and Taiwan [Reference Lian18, 36, Reference Hull37]. Furthermore, in contrast to temperate countries, varicella may have a delayed onset in Hong Kong as in other tropical countries such as India and Singapore [Reference Lokeshwar27, Reference Ooi28]. With the current moderate varicella vaccination coverage in Hong Kong, there is an important implication in the epidemiological shift of varicella to adolescents and adults who are more prone to develop severe disease with poor outcome.
The recommendations from family doctors, specialists and healthcare professionals in school were the most frequently cited factors affecting the parents' decision on whether they should have their children vaccinated with varicella vaccine, while the influence of mass media, relatives and friends were reported to be less important. Our results are in accord with Freeman & Freed's study [Reference Freeman and Freed38], which found that the media were effective in raising public awareness while recommendations from doctors was more influential in the parents' decision. Furthermore, 87·9% of respondents in our study would have their children immunized against varicella if there was community-based promotion by family doctors and healthcare professionals in school, while similar findings have been reported from Taiwan [Reference Liao39]. On the other hand, for those parents who did not have their children vaccinated with varicella vaccine, the most frequently cited barriers were uncertainty regarding the effectiveness of vaccine, lack of recommendations from doctors or government, and adverse side-effects of vaccine, while similar findings have been reported in previous studies [Reference Keane31–Reference Taylor and Newman33].
Our regression model revealed that varicella vaccination could be independently correlated with the socioeconomic characteristics of parents, namely the paternal education level. Similar results have been reported from Taiwan showing that parents with higher education level and higher household income were more willing to have their children immunized against varicella [Reference Liao39]. Willingness to pay for varicella vaccine was significantly associated with varicella vaccination. Parents willing to pay were more likely to have their children vaccinated. In Canada, varicella vaccination uptake rate in regions without public funding for varicella vaccine remained low [Reference Gustafson and Skowronski40], while the uptake rate more than doubled after national varicella vaccination was publicly funded in Australia [Reference Macartney and Burgess41]. Another study from China, where varicella vaccine was only available in private practice, reported a moderate vaccination coverage of 62% which is comparable to our results [Reference Xu42]. Apart from proximity to immunization services (<5 km), county-level economic development was found to play an important role in varicella vaccination. Therefore, consideration of a government subsidy for varicella vaccination for children from lower socioeconomic groups or the implementation of a universal varicella vaccination programme is warranted.
On the other hand, parents' perceived adequate knowledge about varicella and the willingness to seek medical advice for varicella prevention were both positively correlated with varicella vaccination of their children. Surprisingly, our results revealed that a greater perceived risk of varicella was associated with a decreased likelihood of varicella vaccination (OR 0·724), while another school nurse-based study reported the opposite [Reference Salmon43]. These authors argued that despite the high perceived risk of varicella, respondents saw it as a relatively mild disease among other study diseases. Effective strategies in promoting varicella vaccination should therefore focus on not only the perceived risk but also the severity and potential complications of the disease. However, the perceived risk was insignificant and was dropped from the multivariate logistic regression; it might suggest potential interactions between perceived risk and other variables like knowledge on varicella, concern on vaccine effectiveness, etc. Furthermore, the paternal history of receiving varicella vaccine was also found to be a significant factor for the children's vaccination status. From our results, two paternal factors, namely education level and past vaccination experience were important in predicting their children's varicella vaccination status. The father usually plays a dominant role in a Chinese family. With such a socio-cultural setting in Hong Kong, education on varicella uptake should be enhanced to allow mothers and fathers to receive information about the risk and vaccination of varicella.
There are some limitations to our study. First, we recruited parents of children attending kindergartens and thus parents of those out-of-school children might be missed. Since the majority of children attend kindergarten in Hong Kong at the age of around 3 years, it is expected that our sample still bears satisfactory representativeness. Second, our sample was slightly skewed towards the parents and the families with higher education level and higher monthly household income according to census data from the government. Therefore, it might be expected that results from the present study could have underestimated the significance of the parental barriers against varicella vaccination. Third, our findings were limited by the validity of self-reporting measures, which may lead to either under-reporting or over-reporting because of social desirability or conformity. Fourth, varicella is well-known to be an easily recognizable disease but we cannot exclude the possibility of recall bias from the parents on either the varicella history or the vaccination history. Fifth, although the measure of each construct was carefully developed based on concepts from the health belief model [Reference Gustafson and Skowronski40], some other components such as the perceived benefits and barriers against vaccination were not fully explored. Last, as this was an anonymous questionnaire survey, we could not avoid having some parents returning more than one questionnaire if the parents had more than one child whose siblings attended the same or different participating kindergartens. We also could not contact those non-responders to evaluate the reasons for non-response to the survey.
Varicella is an important health problem in Hong Kong, and the varicella vaccination uptake rate needs improvement urgently for optimal control. In order to tackle the major barriers against varicella vaccination, it is strongly recommended that the government and healthcare professional bodies should further enhance health education among healthcare professionals about the significant disease burden of varicella in the community. It is crucial to ensure that healthcare professionals are aware of the importance of their active promotion of varicella vaccination in encouraging parents to have their children vaccinated against varicella. Furthermore, concerted efforts should be made by various stakeholders, including private practitioners, paediatricians, maternal and child health centres, nurseries, kindergartens and schools, in order to enhance parental awareness of the significant morbidity of varicella, as well as the effectiveness and safety of varicella vaccine. With a reduction of unnecessary parental worries concerning the effectiveness and safety of varicella vaccine, the varicella vaccination uptake rate would undoubtedly improve. Local cost-effectiveness data is urgently needed for the consideration of universal varicella immunization in Hong Kong in the near future.
ACKNOWLEDGEMENTS
This research was funded by CUHK School of Public Health and the Primary Care Development Fund. The authors gratefully acknowledge all the participating kindergartens and parents. The authors thank Ms Jasmine Chan and Mr Philip Yeung for their assistance in this study.
DECLARATION OF INTEREST
None.





