Sexually transmitted diseases (STDs) are a major public health concern in both industrialized and developing countries, with syphilis being one of the most common of these diseases [Reference Fenton1–Reference Peterman and Furness4]. Syphilis cases have increased in number in several countries over the last decade, with the highest incidence rates in young adults [Reference Fenton1, Reference Lukehart and Kasper2]. Several syphilis outbreaks were reported in the USA and large European cities, especially in men who have sex with men (MSM) [Reference Fenton and Lowndes3, Reference Peterman and Furness4]. Data from ‘Istituto Nazionale di Statistica’ (ISTAT) [5] and the Italian surveillance system for infectious diseases (SIMI) showed that syphilis cases have increased tenfold from 2000 to 2007, with the greatest increase (92%) in 2000–2002 [Reference Suligoi and Giuliani6]. In the 1999–2004 period, the total number of Italian registered syphilis cases rose from 321 to 1345 [5] but from 2000 to 2006, prevalence rates increased by 146% in those aged 15–24 years and by 199% in the 25–64 years age group [Reference Suligoi and Giuliani6]. Data from the 15–24 years age group in 2005 revealed that latent syphilis was more prevalent in females while males were predominantly affected by primary and secondary syphilis. Syphilis outbreaks occurred in several Italian regions (Lombardia, Lazio, Emilia-Romagna, Piemonte), and especially in large cities such as Milan and Rome, where syphilis infection was more prevalent in HIV-positive men [Reference Cusini, Ghislanzoni and Bernardi7, Reference Giuliani8]. When the STD Centre of Milan detected a significant increase in the incidence rate in 2007 in the region of Lombardia, it raised the alarm to focus public attention on syphilis spread. With this aim, in August 2007, Italian Public Health began promoting a STD-sensitization campaign to raise awareness of the clinical presentation and epidemiology of syphilis. Few epidemiological data on the syphilis-infected population in Tuscany were available and therefore this study was undertaken to ascertain whether prevalence of the infection, sexual behaviour and underlying diseases of cases reflected the wider national picture.
An observational study was carried out in Florence and its province over a 7-year period. Our STD centre within the Infectious Diseases Unit of the Azienda Ospedaliera Universitaria Careggi (AOUC) in Florence collects data from cases from both the city of Florence and the surrounding areas in Tuscany. The study population represents at least half of the entire Tuscan syphilis-infected population, thus reflecting the reality of the disease in the region.
Between January 2003 and December 2009, we examined 259 cases of primary and secondary syphilis acquired by sexual transmission (congenital syphilis cases were not included). All patients were followed by the STD centre of Careggi's Hospital of Florence. The other STD centre in Florence where syphilis diagnosis can be made is at the Dermatologic Clinic II and they collaborated on the project. Patients who tested positive for syphilis were directed to our STD centre by general practitioners, gynaecologists, ER physicians and laboratories or, less frequently, when they showed syphilis lesions. Primary syphilis was diagnosed when patients showed a firm, painless skin ulceration (chancre) localized at the point of initial exposure to the spirochete (e.g. penis, vagina, rectum) and occasional local lymph node swelling [Reference Sparling and Holmes9]. Various manifestations of secondary syphilis were observed; macular syphilis was diagnosed when patients presented with the typical symmetrical reddish-pink (roseola-like) non-itchy rash on the trunk and extremities, typically involving the palms of the hands and the soles of the feet. Patients with intensely pruritic, papular lesions extending over their arms and back were diagnosed as papular syphilis. Many HIV-infected patients showed reddish papules and nodules, some pustular, extending over the face, chest, arms and back. Patients were also diagnosed as secondary syphilis carriers if they presented with one of the following: flat, broad, whitish, wart-like lesions (condylomata lata) localized in moist areas of the body (usually vulva or scrotum); mucous patches on the genitals or in the mouth; pigmentation changes and pustular-ulcerative syphilis; and pharyngitis [Reference Sparling and Holmes9]. We also took into consideration less common clinical presentations such as nail changes, syphilitic alopecia, weight loss, headache, meningismus, fever, sore throat, malaise and enlarged lymph nodes. All serum specimens were first tested for syphilis IgG treponemal antibody using an enzyme-linked immunoassay (ELISA) (Trep-Sure™ EIA, Diamedix, Italy) [Reference Young10]. Specimens with positive or indeterminate ELISA reactions were tested for non-treponemal rapid plasma reagin (RPR) antibodies and those with RPR titre <1:8 underwent confirmatory testing in the Treponema pallidum haemagglutination assay (TPHA) (ASI TPHA test kit, Arlington Scientific, USA) [Reference Young10]. Serum samples with a positive or indeterminate ELISA and an RPR titre ⩾1:8 were considered indicative of recent syphilis infection while those with a positive or indeterminate ELISA, a RPR titre <1:8, and a positive TPHA reflected past infection. Finally, specimens with a negative ELISA or a negative TPHA were considered to be negative for recent or past infection [Reference Sparling and Holmes9, Reference Rahman11]. Serum samples were also tested by fluorescent treponemal antibody-absorption (FTA-ABS) to distinguish recent from previous syphilis infection. All patients were tested for HIV by a screening test ELISA (ADVIA Centaur HIV Ag/Ab Combo, Siemens, USA) and positive results were confirmed by Western blot analysis (HIV Blot 2.2, Genelab Diagnostics, USA) [Reference Guan12].
We collected clinical data (including gender, age and stage of infection), HIV status, information on ethnic background and location where the infection was likely to have been acquired. To estimate the age distribution of primary and secondary syphilis cases were classified by six age groups: 0–15, 16–30, 31–45, 46–60, 61–75 and 76–90 years, and by HIV status. Patients' sexual behaviour, sexual orientation, number of sex partners and condom usage was investigated by means of standardized interviews and a questionnaire. Sex was defined as vaginal, oral and anal, and each patient was asked which he/she thought was the most probable route of transmission for their infection.
According to the stage of infection, 202 (78%) patients had either primary or secondary syphilis and 57 (22%) presented as both forms, with primary and secondary lesions present. The number of syphilis cases varied widely through the years, with a progressive increase of 248% from 2004 to 2008 (Fig. 1). Considering age distribution, 123 (47·3%) patients were aged 31–45 years followed by 45–60 years (75 patients), 16–30 years (37 patients) and there were three patients each in the <15 and >76 years age groups. There were fourfold more males than females (209 vs. 50) and male excess was stable throughout the study period: 72% (2003–2004) 84% (2005), 79% (2006), 94% (2007), 77% (2008) and 81% (2009). Most (80%) males fell within the 45–60 years age group and for females the prevalent age group was 20–30 years. Time-related increment was particularly evident in the period 2006–2008 for both genders. Two-thirds of the male group were Italians, the remainder being foreign immigrants; there were 34 immigrant women (mainly from Eastern Europe and Africa) and 12 Italian middle-aged women.
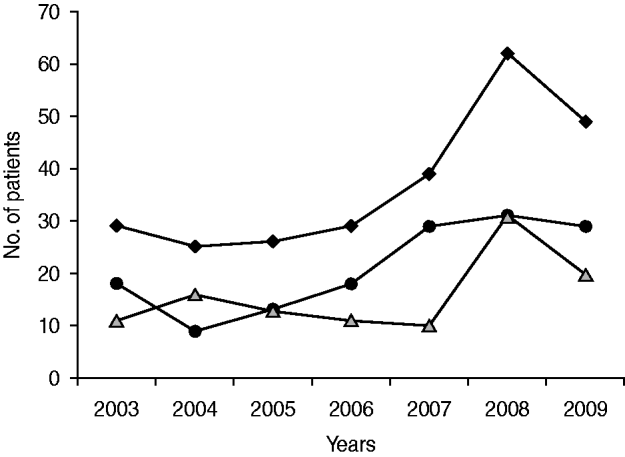
Fig. 1. Total syphilis cases, with and without HIV infection in Florence, 2003–2009. –◆–, Syphilis cases; –•–, HIV-positive cases; ![]() , HIV-negative patients.
, HIV-negative patients.
There were 147 (57%) HIV-positive and 112 (43%) HIV-negative patients. Figure 1 shows that the highest prevalence of HIV/syphilis co-infection occurred in 2007. Between 2004 and 2008, HIV-positive patients increased by 244·4%. Of HIV-positive male patients, about 82% were MSM. It is of particular concern that many patients of the HIV/syphilis co-infected group were still unaware of their HIV-positive status at the moment of clinical observation. HIV infection in these patients (n=85) was detected only during laboratory analysis (CD4+=<200 cells/μl), and were defined as late-presenters [Reference Girardi, Sabin and d'Arminio Monforte13] and represented 32·8% of all HIV-positive patients. Within the late-presenter group, 44 (51·7%) had advanced HIV disease.
The questionnaire and interview survey revealed that 57·4% of the participants had their sexual debut before age 18 years and that rates of unsafe sexual intercourse in the past 6 months were high (70%), especially unprotected oral sex (91%). We assessed that 79·9% of male patients were MSM, 62·2% homosexual and 17·7% bisexual. When asked which type of sexual intercourse they thought to be the most involved route of syphilis transmission, patients answered anal sex in 49% of cases, 37% indicated vaginal sex and 14% oral sex. We suggest on the basis of clinical examination and history that oral sex was the probable cause of transmission in 70% of all patients, in 80% of MSM and in 73% of HIV-positive patients.
The inflection point observed in 2008 and 2009 when cases decreased from 69 to 42 was likely to be related to the STD-sensitization campaign promoted by Italian Public Health since August 2007. Risk behaviours which lead to syphilis transmission are: a high number of concurrent sexual partners, sexual intercourse with partners of unknown HIV status or with commercial sex workers which also predispose to the infection. In addition, online social networks have provided a rapid and easy way to meet multiple sex partners (of unknown HIV status), especially in MSM [Reference Peterman and Furness4, Reference Elford14]. These changes in sexual behaviour, along with travel and migration from high-prevalence syphilis areas, have helped the re-emergence of syphilis more than biological susceptibility [Reference Fenton1–Reference Peterman and Furness4, Reference Elford14]. Based on the sexual behavioural characteristics of our case group we conclude that disinformation and ignorance of the basic epidemiology of the disease was the main cause of syphilis transmission. Indeed, our survey suggests that oral sex played an important role in syphilis transmission as many patients did not think oral sex could be a route of STD transmission and were very surprised when diagnosed with syphilis [Reference Fenton1, Reference Peterman and Furness4]. These patients included HIV-positive MSM who practised oral sex thinking it was safe. Early sexual debut was also a common risk factor and four cases of acquired syphilis in 14-year-old Florentine girls were found between 2008 and 2009. Our study confirms several other reports of the higher incidence of syphilis in HIV-infected patients [Reference Fenton1, Reference Fenton and Lowndes3, Reference Peterman and Furness4, Reference Cusini, Ghislanzoni and Bernardi7, Reference Giuliani8]. The reasons for this co-infection predominance are not completely clear, but may be related to the unprotected high-risk sexual behaviour common in the HIV-positive MSM population. In this regard, it was particularly interesting to note that, as highly active antiretroviral therapy (HAART) became available, the number of syphilis cases has risen in HIV-positive people, probably because, as the fear of AIDS lessened, unsafe sex practices increased [Reference Battegay15].
Interestingly, one third of our HIV/syphilis co-infected patients turned out to be late-presenters with HIV, half having advanced HIV disease which came to our attention due to the manifestations of syphilis alone, and who were unaware of their HIV infection. We therefore endorse the view that syphilis may herald the presence of HIV and thus allow earlier diagnosis of HIV [Reference Elford14]. Several studies have reported high prevalence rates for syphilis in HIV-infected MSM [Reference Fenton1, Reference Fenton and Lowndes3, Reference Peterman and Furness4, Reference Cusini, Ghislanzoni and Bernardi7, Reference Giuliani8]. Moreover, HIV-infected MSM play an important role in bridging the transmission of HIV, syphilis and other STDs from their high-risk male sexual partners to low-risk female partners [Reference Fenton1].
We conclude that laboratory screening tests for syphilis should be performed regularly for MSM (HIV-positive, negative and unknown status) who have at least one partner in the year. This combined with epidemiological surveillance and monitoring might help to reduce the burden of this disease and give further insights into the risk factors for syphilis transmission in HIV-infected people in large urban settings.
ACKNOWLEDGEMENTS
The authors thank the Microbiological and Virological Serum-Immunology Laboratory of the Azienda Ospedaliero Universitaria Careggi-AOUC for technical help.
DECLARATION OF INTEREST
None.





