Background
Differences in health and susceptibility between men and women have been studied in various medical contexts that go beyond reproductive areas of biology. Existing research in this context aims to disentangle biological mechanisms as well as environmental and behavioural drivers and thereby contributing to effective preventive and curative interventions. Infectious disease incidence is influenced by individual factors such as hormonal effects that make men more susceptible [Reference Klein1] and by basic genetic and physiological constitutions and different immune responses [Reference Witzemann and Pardue2]. Preventable infections, in particular, are additionally influenced by health-related behaviour, resulting in different utilisation of preventive interventions such as vaccines or varying exposure to, e.g. occupational and environmental hazards. These factors, in turn, are directly or indirectly modulated by age. The complexity of interpreting sex- and age-patterns of infections is illustrated by the example of influenza and pneumococcal vaccination. For both, the immune responses and post-vaccination protection have been shown to interrelate with modifiable behavioural factors like smoking [Reference Cruijff3, Reference Sankilampi4] which in itself is unevenly distributed across sexes and ages.
Few efforts have been undertaken to systematically analyse sex- and age patterns across a range of infections. Furthermore, gender-specific prevention strategies have mostly been promoted [Reference Stahlman5] and studied [Reference Ehrhardt6] with regard to sexually transmitted infections. One publication addressed sex-and age-specific patterns of ten selected notifiable infections using compulsory notification records from Brazil [Reference Guerra-Silveira, Abad-Franch and Nishiura7]. Based on the restricted and pre-selected number of infections the study concludes that, in contrast to physiological factors, behavioural differences only play a secondary role and do not generally explain strong sex- and age variations. This, however, does not seem to concur with study findings that demonstrated the significant impact of gender-specific interventions on risk behaviour such as preventing sexually transmitted infections [Reference Ehrhardt6]. Given the lack of comparably available literature and the importance of sex- and age differences in incident infections with regard to their prevention, our objective was to identify patterns of such specific occurrences across infections. This was realised by analysing the incidence of notifiable infections in Germany over a 13-year time frame (2001–2013) with regard to sex-specific occurrences and to evaluate the extent to which these are influenced by age at notification.
Methods
The German infectious disease control act (Infektionsschutzgesetz, IfSG) regulates mandatory surveillance and case definitions for notifiable infectious diseases. An electronic surveillance system is in place to support the communication of notifications between local, federal and state institutions; the system also consists of an electronic outbreak reporting system [Reference Krause8]. A total of 378 local health departments in Germany verify locally identified notifiable diseases with reference to national case definitions and send case reports electronically through the 16 state health departments to the national surveillance unit at the federal institution responsible for infectious disease surveillance in Germany (Robert Koch Institute (RKI)) (§6 and §7(1) IfSG and state-specific regulations). Reports on HIV, Treponema pallidum, congenital toxoplasmosis, congenital rubella infection and Plasmodium falciparum are sent directly from laboratories to the RKI (§7(3) IfSG). According to procedures of the German national surveillance system, haemolytic uremic syndrome (HUS) and Escherichia coli enteritis are mutually exclusive categories. As for most other pathogens, laboratory detections of notifiable infections without a clinical manifestation, as defined in the respective case definition, do not contribute to the case count. Data are transmitted to the RKI without information making the individual identifiable [Reference Krause8]. A database on notifiable diseases reported in Germany is maintained by the RKI (https://survstat.rki.de/). This analysis is based on individual reports of all notifiable infections which (a) match the respective case definitions mandated by the IfSG, (b) were incident cases and (c) were reported from 2001 to 2013 to the RKI, by 01.03.2014. We used fully anonymised case reports including variables such as: name of pathogen or infection/disease resulting from infection with a pathogen (-group), age in years, sex, year and week of notification, county and state of residence. Due to the nature of surveillance data, individuals with multiple notifiable infections acquired through the 13 year observation period appear as multiple cases. For computation of incidence from available individual reports, we used sex- and age-specific population data corresponding to the year in which the case was notified. This information was used as the denominator to calculate the incidence. At the time of data analysis, stratified population data were not yet available for 2012 and 2013; hence the 2011 population data were used for cases notified in 2012/13. There is no indication that the German population data have changed substantially between 2011 and 2013.
We excluded pathogens/infections, which were not continuously reported between 2001 and 2013 (except for Methicillin-resistant Staphylococcus aureus (MRSA) due to its high public health relevance, which was introduced to be notifiable in the year 2009). Rare infections with <1000 notifications over the 13-year time period were also excluded as were notified cases with missing information on sex and/or age.
We calculated age-standardised incidence rates per 100 000 population for each pathogen/infection. These were calculated cumulatively over all 13 calendar years and for both sexes for 15 age-groups (0, 1, 2, 3, 4, 5–9, 10–14, 15–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79 and ⩾80 years) by aggregating the number of cases and using 2007 German population data (median year) as denominator. We used 1-year intervals for young ages due to observed variations in child age for some pathogens/infections. For Poisson regression analyses, age-standardised incidence rates were entered as dependent variables and sex as the independent variable. We generated a male:female incidence rate ratio (IRR) for each pathogen/infection for 15 age-groups, and corresponding 95% confidence intervals (CI) at a significance level of α = 0.05. We further combined the number of cases to generate broader age-groups and obtain IRRs for ages 0–4 (infants), 5–14 (children), 15–59 (juveniles and adults) and ⩾60 years (elderly) as well as additionally for the age group that overarches ages 0–14 years. IRRs >1 indicate a male dominance with those below 1 indicating a female dominance. We consider IRRs with CIs crossing 1 to be equal for males and females. We categorised pathogens/infections according to similar sex-specific incidence patterns, under consideration of IRR variation by age-group. To obtain a combined IRR for each age category within the identified pattern and therefore check the consistency of sex patterns by age category, we applied Poisson regression overarching all pathogens of the respective group. We assessed possible overdispersion in the Poisson regression models by performing negative binomial regression analysis and likelihood ratio tests of alpha values. We assessed incidence variations over time by comparing male:female incidence ratios using year-specific information. All statistical analyses were done using STATA© 12.0.
Results
The analysis encompasses 3.96 million records of 33 notifiable pathogens or infections received between 2001 and 2013 (2 004 407 males and 1 955 593 females). Overall, infections with norovirus, campylobacter and rotavirus were most frequently reported. Among females, norovirus was most commonly notified (n = 505 768), whereas among males, campylobacter was the most common pathogen (n = 411 211). For the majority of pathogens, incidence rate ratios show an overall male preponderance. A few diseases were significantly more frequent in females, specifically in those aged 15–59 years. Among them were E. coli enteritis (IRR: 0.78, 95% CI 0.62–0.97), infections with the enterohemorrhagic E. coli (EHEC) (IRR: 0.66, 95% CI 0.46–0.95), norovirus (IRR: 0.75, 95% CI 0.71–0.79) and rotavirus (IRR: 0.66, 95% CI 0.60–0.73) infections as well as salmonellosis (IRR: 0.93, 95% CI 0.88–0.94). The highest IRRs (corresponding to a male predominance) were seen for syphilis with men aged 15 years and older being over nine times more afflicted than women (Table 1). These results were robust when assessing for variations in incidence over time. The regression analyses revealed no statistically significant difference between males and females for: adenovirus infection, hepatitis A virus (HAV) and hepatitis E virus (HEV) infection, Haemophilus influenza b (HIB) infection and for diseases such as Creutzfeldt-Jakob disease (CJK/CJD), Q fever, cryptosporidiosis, dengue fever, echinococcosis, HUS, measles and shigellosis. Including IRRs for those infections that indicated significant differences in incidence by sex as obtained from the Poisson regression, and under subsequent consideration of age, five patterns of similar sex/age incidence became apparent (Tables 2 and 3):
Table 1. Pathogen-specific male:female incidence rate ratios with 95% confidence intervals, by age groups

* P < 0.05.
Table 2. Patterns of sex and age of notifiable infections with significant sex difference
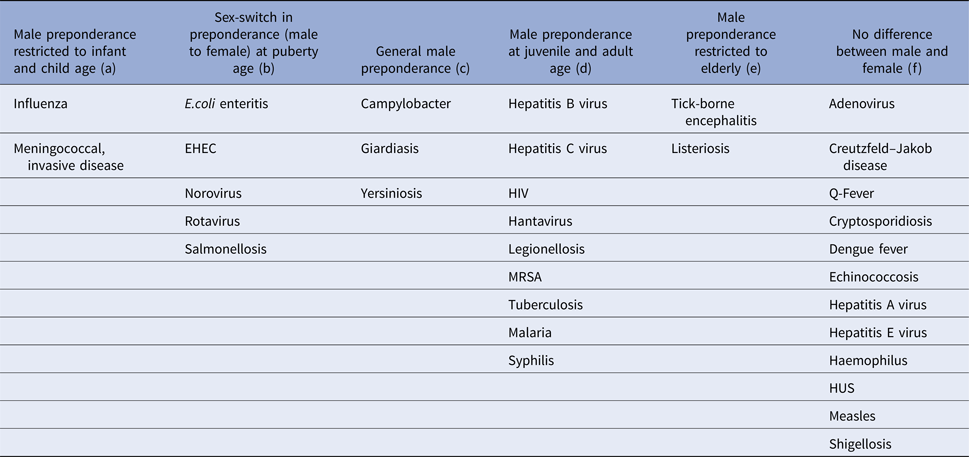
Table 3. Age-group-specific IRRs across infections of identified patterns
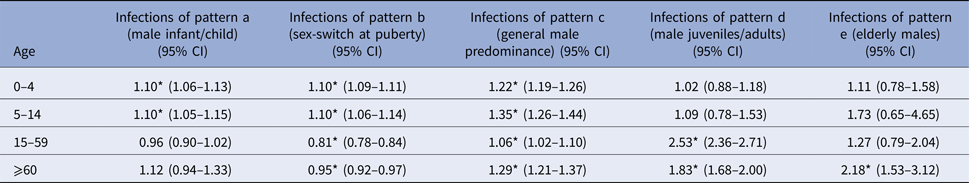
* P < 0.01.
(a) Infections with male incidence preponderance restricted to infant and child age
Influenza and meningococcal disease caused by Neisseria meningitidis affect males at infant and child age more frequently than females of the same age. For invasive meningococcal disease, this effect was significant among 0–4-year old boys (IRR = 1.19, 95% CI 1.03–1.38), and for the overarching age-group 0–14 years (IRR = 1.18, 95% CI 1.02–1.36). Influenza incidence showed a male dominance for age-groups 0–4 years (IRR = 1.09, 95% CI 1.06–1.13) and 5–14 years (IRR = 1.10, 95% CI 1.05–1.15) separately as well as for the overarching age-group 0–14 years (IRR = 1.09, 95% CI 1.07–1.12).
(b) Infections with sex-switch in incidence preponderance at puberty age
This group encompasses pathogens and infections, which are food-born and includes E. coli enteritis, salmonellosis, infections with the enterohemorrhagic E. coli, norovirus and rotavirus. Most of these show a significantly higher incidence in males compared with females up to the age of 14 years, followed by a reversing relation from 15 years of age onwards, when females were more often affected than males. This pattern is consistent in all of these pathogens, but in some age groups the respective difference does not reach statistical significance, e.g. for rotavirus (male dominance not significant for 5–14-year old males) and salmonellosis (male dominance not significant in oldest and youngest age group).
(c) Infections with general male incidence preponderance
Throughout all age groups men tended to be more often afflicted than females by infection with campylobacter, Giardia and Yersinia (Table 3). For campylobacter this difference was significant for all four age groups (Table 1), for giardiasis for the first (0–4 years) and the third (15–59 years) age group, and for Yersinia for all but the oldest age group (60+ years).
(d) Infections with male incidence preponderance at juvenile and adult age
Legionellosis as well as sexually transmitted and vector-borne infections including hepatitis B virus, hepatitis C virus and HIV infection, malaria, dengue fever, hantavirus infection, tuberculosis and MRSA infection show a trend of male dominance at juvenile and adult age (⩾15 years). These infections were rare among children in general and peaked among men at middle age with men aged 15–59 years being 1.94 (95% CI 1.73–2.18), 2.54 (95% CI 1.75–3.67) and 2.19 (95% CI 1.53–3.15) times more likely to be notified with hepatitis C virus infection, hantavirus infection and malaria, respectively, compared with women. In addition to a significantly higher risk of males aged 15–59 years, hepatitis B virus, HIV, MRSA and TB infection as well as legionellosis and syphilis also affected males at an older age (⩾60 years) significantly more often than females in this age- group.
(e) Infections with male incidence preponderance in the elderly
Tick-borne encephalitis and listeriosis showed males aged 60 years and older to be over two times more likely to be affected than females in this age group (IRR of 2.75, 95% CI 1.21–6.24 for tick-borne encephalitis and IRR 2.06, 95% CI 1.38–3.06 for listeriosis). There was no significant difference between males and females for these infections in all other age groups.
Discussion
Our analyses revealed five sex/age patterns for 21 pathogens/infections, based on IRRs that demonstrated significant sex differences. For additional 12 infections, no statistically significant sex differences were detected. It is well known that for most notifiable diseases, in particular for sexually transmitted and blood-borne infections, males have significantly higher incidence rates than females [Reference Gilsdorf9]. However, sex differences are also found for most pathogens with other transmission routes and have been rarely discussed in the literature. Such differences do not necessarily apply across age groups. For example, in our analyses that considers age in addition to sex, the overall higher incidence of E. coli enteritis in males compared with females was only significant in the infant age group and reversed from age 15 years onwards, when females had a higher infection incidence than males. Other pathogens/infections that showed a pattern of switch in sex dominance at puberty age (pattern b) encompass food-borne diseases such as EHEC, rotavirus gastroenteritis and norovirus gastroenteritis. Explanations for this pattern can relate to both, behavioural and intrinsic factors such as hormonal differences that appear at puberty and can impact on susceptibility to clinically relevant infection. Regarding behaviour and lifestyle, it has been stated that women at adult age have a higher exposure to food-borne virus infection due to their frequent presence in settings such as hospitals and day-care centres where e.g. norovirus is transmitted [Reference Patel10]. Women of reproductive age could also be more likely than men to represent secondary cases if they were infected by their children. Whether this quantitatively explains the difference has to our knowledge not been assessed. Furthermore, it raises the question why campylobacter infection does not follow this pattern given its similarities regarding transmission route and exposure-related determinants. The male dominance at a young age could be related to biology that results in a more vulnerable immune system of young males compared with females. The biological mechanisms involved in the immune system and immune response are mainly of hormonal origins such as the correlation of high testosterone levels with reduced immune responses [Reference Furman11] or the protective effect of oestrogens against infections [Reference Klein1]. As testosterone levels in males rise shortly after birth, the so-called ‘mini-puberty’, and decline after [Reference Ober, Loisel and Gilad12], new-born males might be more susceptible to infectious diseases in general and food-borne agents in particular.
The theory of intrinsic factors would thus also explain the high incidence in infant males relatively to female infants for influenza and invasive meningococcal disease which follow the same sex-age pattern (pattern a). The higher female incidence after puberty may accordingly be related to endogenous or exogenous hormonal changes affecting women at childbearing age and potentially impacting on their vulnerability to infection. It might also be a consequence of the above mentioned behavioural contributors to exposure. Interestingly, for invasive meningococcal, the statistically significant male dominance in the age-group 0–14 seems to be driven by an increased risk for males aged 0–4 years, compensating for the non-significant trend among 5–14-year olds.
Another finding of our study also contradicts common concepts of risk factors: Food- or water-borne infections do not consistently follow the same sex/age-incidence pattern. I.e. those infections resulting from bacteria (pattern c) including campylobacter or Yersinia enterocolitica affect males throughout life more frequently than females, which is different from the food-borne infections with a switch in sex dominance (pattern b). Infections with campylobacter or Yersinia are attributable to various sources of exposure, e.g. consumption of contaminated meat [Reference Wingstrand13, Reference Rosner14] or drinking water [Reference Braeye15]. Another major transmission route for campylobacter is contacted with farm animals, e.g. through occupational exposure. More frequent consumption of fresh poultry or specific male occupations could result in a higher risk of men. However, this behaviour and exposure-related assertion would not serve as a primary explication in light of research, which showed that higher exposure to these bacteria increase the development of immunity. Higher C. jejuni antibody levels would thus rather prevent males from becoming ill [Reference Blaser, Sazie and Williams16, Reference Jones, Robinson and Eldridge17].
The group of infections with a preponderance in juvenile and adult men (pattern d) encompasses blood-borne, sexually transmitted infections, nosocomial, vector-borne and/or travel-related diseases. For those typically transmitted through blood (e.g. intravenous drug use), the higher infection rates among males at a sexually active age-span are easily explained by behaviour [Reference Stahlman5, Reference Leigh and Stall18, 19]. In addition, implemented sex-specific prevention practices such as syphilis-screening or hepatitis B screening of pregnant women may have resulted in effects that manifest in a lower incidence among adult females compared with males. The vector-transmitted and/or travel-associated diseases following the sex-age pattern of group ‘d’, namely malaria and hantavirus infections can also mostly be explained by behaviour and subsequent exposure. Malaria is well studied with regard to sex differences and explanations are in part applicable to other ‘pattern d infections’. While in malaria-endemic settings various physiological characteristics have been attributed to males being more frequently and more severely afflicted by malaria [Reference Stienlauf20, Reference Schlagenhauf21], in a setting where malaria is almost exclusively imported, these factors are likely to be less relevant. The allocation of malaria into the group of infections with a strong behavioural risk component makes the specific male risk behaviour most explanatory for the sex differences. This behaviour may include a lower adherence to protective measures [Reference Stienlauf20–Reference Phillips-Howard23] and a higher likelihood of travelling to malaria-endemic areas [Reference Stienlauf20, Reference Schlagenhauf21]. However, in searching for specific explanations for the male preponderance for malaria and hepatitis B virus infection, one has to take into consideration that a large proportion of notifications could have occurred among migrants from malaria-endemic countries, which in turn are more likely to be males. Although the German surveillance system does not capture the country of origin of notified individuals, it records the countries of origin of the infection in notified individuals. These countries were to a vast majority those located in Africa [Reference Schöneberg24], which are also countries of origin of many migrants. Thus, it could be hypothesised that the male preponderance among malaria cases notified in Germany is strongly influenced by the male preponderance among the migrant population coming from affected countries. This would also explain why we did not find a significant difference in incidence between males and females for dengue fever, a result which is also in line with previous findings [Reference Stienlauf20, Reference Jelinek25]. Lack of adherence to protective measures and travelling to high-risk areas were also not affected by sex in another study but pre-clinical travel advice seeking was significantly more common among females [Reference Schlagenhauf21] which would support the theory of behaviour being the most relevant explanation for all group d infections and would thus allude for a need of enhanced sex-specific prevention measures that target middle-aged males. The higher male incidence for legionellosis reflects a sex pattern that is also observed from legionellosis outbreaks [Reference García-Fulgueiras26] and reasons can relate to higher susceptibility due to a higher prevalence of behavioural risks such as smoking [Reference Nguyen27] and alcohol consumption [Reference Bloomfield, Kraus and Soyka28–Reference Lampert, Lippe and von der Müters30], but possibly also genetically determined differences in susceptibility. The difference for MRSA has also been seen in prevalence studies on S. aureus which did not identify specific behavioural or exposure-related explanations [Reference Mehraj31]. Similar to HIV and syphilis, the male preponderance for hepatitis B virus infection could be a consequence of a higher frequency of risk-related male behaviour, including injecting drug use or sexual transmission among men having sex with men.
Pattern ‘e’ includes diseases with a significant preponderance in males of older age. For tick-borne encephalitis, this is well documented [Reference Lindquist and Vapalahti32] and may be due to higher recreational exposure to tick environments but the identified strength of the IRR remains of surprise. Even more difficult to explain is the marked male preponderance at an older age for infections caused by Listeria, particularly since it is not similarly strong in the younger age group. A clear explanation for the sex–age pattern of listeriosis shared with tick-borne encephalitis is not obvious. A higher awareness of the disease risk among older females might be possible, e.g. through sensitisation for the fact that listeriosis is most risky if acquired during pregnancy.
Strength and limitations
For the creation of sex-specific patterns under consideration of age for susceptibility to infections, we used data from 13 years of statutory surveillance of a population of around 82 million individuals and calculated standardised ratios using population data stratified by age, sex and year. This makes our analysis and findings fairly robust against temporal variations and possible biases. While underreporting is a known limitation in surveillance systems, it is unlikely to distort our findings in a relevant way since our aim was to investigate relative differences in incidence between sexes and not absolute differences. Although a sex-specific selection and information bias can affect statutory surveillance [Reference Thacker and Berkelman33] this is less likely in countries like Germany, where the structure of the health insurance system minimises sex-specific difference in access to diagnostic service for infections. Furthermore, if such an effect would play a significant role, it would be difficult to explain why it should play towards preponderance of a particular sex in a particular disease and age group and not in another, as presented by our data. Nevertheless, the nature of the data does not allow quantifying how much of variation in infectious diseases incidence by sex is due to social/behavioural factors of the diseased person like health-seeking behaviour that is influenced by e.g. presence of young children in the household and by physician behaviour. Factors can derive from sex-specific perception of pain and symptom recognition and can influence sex-differences in reporting of infections [Reference Nasirian34]. Furthermore, it is likely that for some unique infections such as malaria or Hepatitis B, distortion is induced by the composition of cases, which could be mostly migrants and which may have a sex-distribution that differs from the general German population. Except for tuberculosis [Reference Bales35] the German surveillance system does not capture the country of origin of notified individuals [Reference Schöneberg24], so that this aspect cannot be taken into account across pathogens.
Since we based our analysis on infections with case counts of >1000 and with consistent reporting throughout the years, changes in notification regulations made it necessary to exclude avian influenza (notifiable since 2007), pertussis, mumps, rubella (notifiable since 2013), severe Clostridium difficile infections (notifiable since 2009) and varicella (notifiable since 2013). We also identified a number of pathogens with no significant sex difference in incidence according to IRRs (adenovirus, HAV, HEV, HIB, CJK/CJD, Q Fever, cryptosporidiosis, dengue fever, echinococcosis, HUS, measles and shigellosis). Some of the explanations provided for the five patterns could also apply to these pathogens but are difficult to disentangle due to multifactorial risk factors occurring simultaneously. The non-significant difference between males and females for some of the pathogens could also be a consequence of low overall case counts in the respective age-strata.
In addition to routine annual reports generated for notifiable diseases, our analyses illustrate that frequency and intensity of exposure to disease-causing pathogens not only varies by sex but can create reverse pictures when looking at particular age-groups and when adjusting for the age-specific distribution of the population at risk. This demonstrates differential exposure to risk and protective factors associated with sex but also during certain life stages. These factors may include behavioural and occupational hazards, humoral and anatomic disposition. The grouping of pathogens/infections in some of the patterns identified in our analyses has common and accepted explanations. For example, sexually transmitted diseases affecting males at juvenile and adult age have a clear behavioural risk component, which has been subject to sex-sensitive prevention approaches [Reference Ehrhardt6]. Other findings are of surprise and explanatory evidence is not available from our data, as for example in the case of MRSA, which shows a pattern of preponderance among elderly males. However, our findings indicate that effects of some commonly addressed risks and prevention approaches may overall not be sufficiently strong to manifest in data, e.g. some vaccinations targeting both sexes equally. This underscores that susceptibility to infections is influenced by a complex interaction of multiple factors – some of which are not even fully understood. Our findings may trigger investigations particularly for genetic determinants of susceptibility to specific infections as well as on epidemic-prone diseases which have been studied rarely with regard to their gender-related transmission and susceptibility aspects [36]. The study confirms that for many notifiable infections males are at higher risk than females but that this varies when considering age in the analyses. The approach to identify groups of infection that follow similar patterns in occurrence according to two core factors (age and sex) is unique and can add to the so far lacking evidence on gender-related aspects of infectious diseases, which are crucial for effective public health efforts, including outbreak control [36]. Results suggest a need for gender-sensitive prevention efforts that target infections in a broader scope than limited to sexually transmitted diseases.
Acknowledgements
We would like to thank André Karch for his expert advice and guidance on the statistical analyses.
No external funding. Staff salaries of J.J. Ott and G. Krause derive from the Helmholtz-Centre for Infection Research. Staff salary of H. Claus derives from the Robert Koch-Institute. F. Walter is a student and conducted the work as part of his Master Thesis.
Conflict of interest
None.
Authors contributions
J.J. Ott led the writing of the paper, presented the results and wrote the paper together with F Walter. F Walter developed and performed the statistical analyses in collaboration with J.J. Ott. H Claus provided the original data and expertise on official notification rules and data; he commented on the manuscript. G Krause created the project idea and initiated the project. He supervised the study, guided and contributed to the interpretation of obtained results. All authors contributed to the manuscript and approved the final version for submission.





