Mammalian reoviruses (respiratory enteric orphan viruses), members of the family Reoviridae, are non-enveloped, double-stranded (ds) RNA viruses with a genome composed of 10 segments. Although orthoreoviruses have been identified as the causative agents of diseases in animals, infections in humans are generally benign, only rarely causing cases of mild upper respiratory tract illness or enteritis in infants or children. Nevertheless, many studies have reported reovirus isolation from patients with meningitis [Reference Tyler1], acute necrotizing encephalopathy [Reference Ouattara2], or acute respiratory tract infection [Reference Jiang, Hermann and Coombs3–Reference Cheng6]. Tyler et al. detected reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts [Reference Tyler7]. We previously isolated a new type of reovirus from a severe acute respiratory syndrome (SARS) patient, which was the first case in Beijing, China [Reference Duan4], which we designated R4. Since only a few seroepidemiological studies on reoviruses have been reported, we determined the titres of antibodies specific for R4 in sera from healthy people and patients in our hospital.
In this study, R4 [Reference Duan4] was used during quantification of the levels of the relevant neutralizing antibody by a microneutralization assay. Blood samples were inactivated at 56°C for 30 min and were then diluted at a ratio of 1:2, for a series of dilutions (1:4–1:256); 50 μl of the serially diluted serum was mixed with an equal volume of 100 TCID50 of R4 and the samples were added to a 96-well microplate (NUNC, Denmark), which was incubated at 37°C for 2 h. L929 cell suspension (1 × 105 cells/ml) was added to each well. A cell control, serum control, virus control, and virus back-titration (if the result for the backdrop test was 32–320 TCID50/well, the test was considered successful) were included with each plate. Plates were incubated in a CO2 incubator at 35°C for 7 days. Cytopathogenicity was observed by microscopy to identify titres of neutralizing antibodies, which was defined as inhibition of 50% of the cytopathogenic effect. Neutralizing antibodies were considered positive if the dilution was ⩾1:32.
Serum samples of 219 patients with measles, hand-foot-and-mouth disease (HFMD), liver disease, or diarrhoea were included (Table 1). Sera from 97 blood donors were investigated as controls. Of the 316 subjects [mean age (±s.d.) 30·95 ± 19·05 years], 57·59% were male and 42·41% were female. The neutralization assay indicated that the median antibody titre was 1:64 for the HFMD group, 1:128 for the liver disease group and the measles group, and 1:256 for the diarrhoea group, vs. 1:32 for blood donors. The total positive rate in patients significantly differed from that in blood donors (85·39% vs. 29·90%, P < 0·01; Fig. 1 a). The positive rate in blood donors was significantly lower than those in the other four groups (P < 0·01). Of the patients, the positive rate in the HFMD group was lower than those in the other three groups and significantly differed from those in the liver disease and diarrhoea groups (70·8% vs. 90·2% and 100%, respectively; both P < 0·05). The measles group had a positive rate similar to that of the other three groups (P > 0·05).
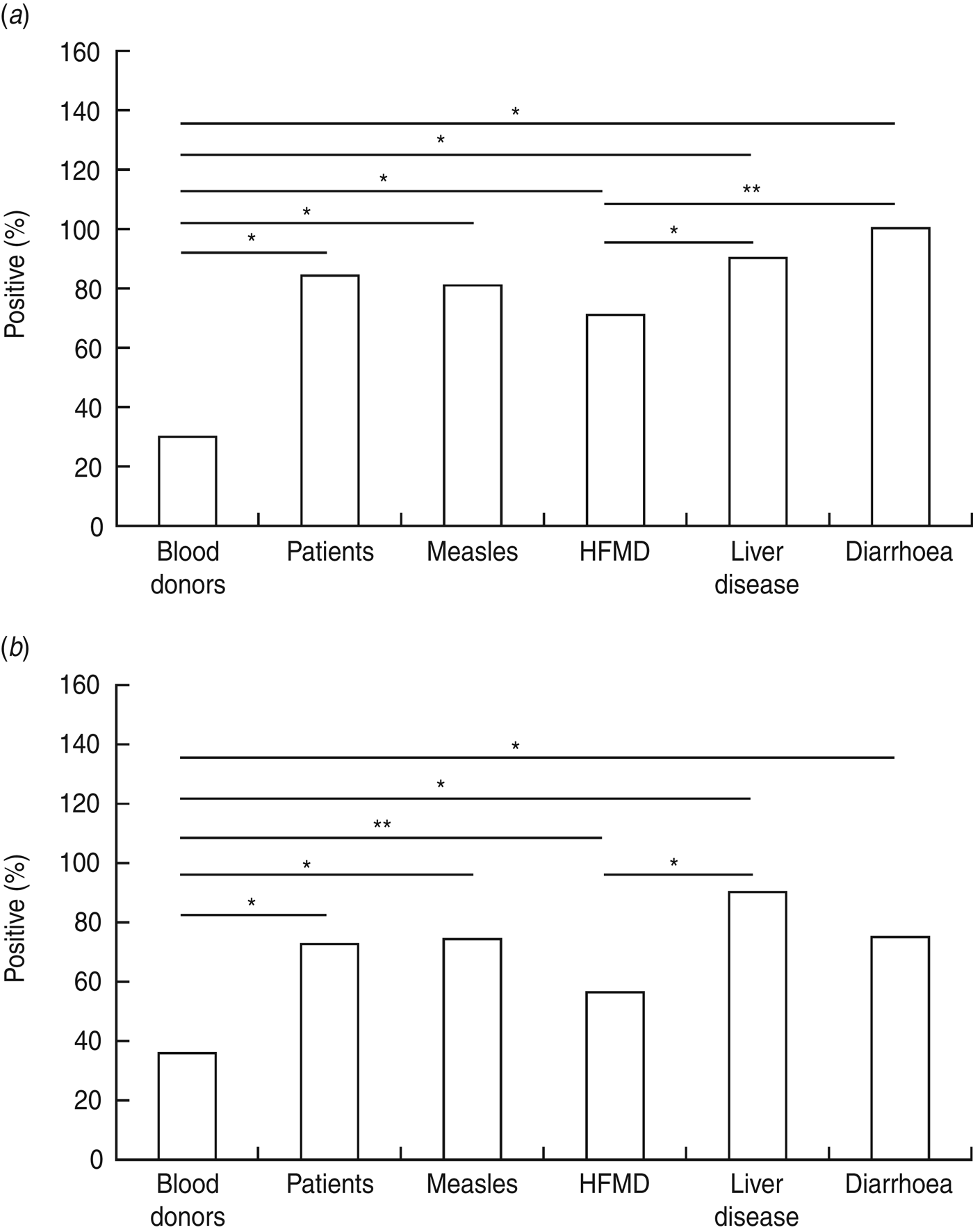
Fig. 1. (a) Comparison of positive rate of microneutralization assay for R4. Differences in positive rate were analysed in groups by χ 2 test using SPSS v. 12.0 software (SPSS Inc., USA) (*P < 0·01, **P < 0·05). (b) Comparison of positive rate of ELISA assay for R4. Differences in positive rates were analysed in groups by χ 2 test using SPSS v. 12.0 software (*P < 0·01, **P < 0·05).
Table 1. Demographic and clinical information of the patients recruited – meals, HFMD, liver diseases and diarrhoea

HFMD, Hand-foot-and-mouth disease.
* Patients with a positive result.
To confirm the result of the neutralization test, ELISA was performed to detect the antibody of σ1 protein (encoded by the s1 gene of the reovirus) in these populations. We chose 10 serum samples from the healthy population that had been negative for the microneutralization assay as the negative control. σ1 protein was expressed by IPTG induction and the inclusion bodies were taken as coated antigen (100 ng/well). After coating the 96-well microplates at 4°C overnight and blocking at 37°C for 3 h [blocking buffer, 30% fetal bovine serum in phosphate-buffered saline (PBS)], the serum was diluted 1:100 with PBS and incubated at 37°C for 1 h; detection was performed with a 1:5000 dilution of horseradish peroxidase-conjugated goat anti-human IgG (H+L) (ZSGB-BIO, China). After washing, the plate was incubated with 3,3′,5,5′-tetramethylbenzidine (Sigma, USA) for 15 min and colour development was stopped by adding 2 m H2SO4. The optical density (OD) value at 450 nm was read. For each sample, we calculated the OD value of the antigen-coated well and subtracted the value of the corresponding control well to obtain the corrected OD. A sample was considered seropositive when P/N > 2·1, where P = corrected OD, and N = mean OD of negative controls.
For 52 patients with liver disease, the serum samples were insufficient to allow the use of both detection methods; therefore, only 264 serum samples were included in the ELISA assay. The results of the ELISA (Fig. 1 b) were consistent with those of the neutralization assay, except that the difference between the HFMD and diarrhoea groups was insignificant (P > 0·05). Moreover, for each group, the positive rate did not significantly differ between the two detection methods.
Seroprevalence for R4 was not significantly different between males and females in each group, excluding the blood donor group, by ELISA detection (23·81% vs. 45·45%, P = 0·028). Additionally, the difference in R4 prevalence in populations from northern China, southern China, eastern China, and northeastern China was not significant (data not shown). We also investigated the correlation of antibody levels and the number of lymphocytes in each group. Only patients with liver disease and HFMD showed a negative correlation between lymphocyte numbers and antibody titres, with correlation coefficients of −0·409 (P = 0·025) and −0·293 (P = 0·043), respectively. Furthermore, no correlation was observed between the patient's age and antibody titre (P > 0·05).
Mammalian reoviruses are common infectious agents in humans. Determining the prevalence of reovirus-specific antibodies can help analyse proposed but unproven associations between reovirus infection and clinical diseases (e.g. neonatal extrahepatic biliary atresia and meningitis) [Reference Tyler1, Reference Ouattara2]. The present study investigated the seroepidemiology of R4, which we recently isolated in healthy people and patients reporting to our hospital; the R4-specific antibody titres and the positive rate were higher in patient groups than in healthy controls. It was unclear whether the presence of other disease or infective agents could enhance the infectivity of R4, thus aggravating its pathogenicity or exacerbating the disease condition. This could be due to a synergistic effect, as described by Piccoli et al. [Reference Piccoli8], who demonstrated that synergy between hepatic injury and reovirus significantly increased the magnitude of viral infection and contributed to mortality. Although reovirus is regarded as a non-pathogenic agent and has not been associated with a specific clinical syndrome, it has been reported that reovirus strains have been isolated from persons with serious human diseases [Reference Tyler1–Reference Cheng6]. Many other reovirus strains have also been isolated from patients with acute respiratory syndrome [Reference Jiang, Hermann and Coombs3–Reference Cheng6] and from children with acute necrotizing encephalopathy [Reference Ouattara2]. Therefore, we believe that the presence of R4 antibody in patients should receive more attention.
However, R4 antigens in serum samples were not detected by RT–PCR, and no virus was isolated by viral culture. This could be explained by the possibility that a high level of neutralizing antibody was induced by subclinical reovirus infection early in life [Reference Tai9] or by exposure to a contaminated environment [Reference Kim, Shin and Kim10] or that the viral antigen had been cleared while the antibody was retained for some time after the immune response.
Although the study population was limited, our data suggest that R4 is spread widely throughout the human population. A significantly higher level of R4-specific antibody in patients than in healthy people warrants consideration, since it poses a risk for aggravation of the extant illness by the reovirus.
ACKNOWLEDGEMENTS
This work was supported by the National Nature Science Fund, China (grant no. 81000735).
DECLARATION OF INTEREST
None.




