INTRODUCTION
Alveolar echinococcosis (AE) is a zoonosis caused by the larval stage of the fox tapeworm, Echinococcus multilocularis. Humans serve as an intermediate host for the cestode and actually are an inappropriate or ‘dead-end’ host [Reference Ammann and Eckert1, Reference Gottstein2]. Almost without exception, the primary site of disease manifestation in humans is the liver [Reference Ammann and Eckert1, Reference Reuter3]. More than 10 years may pass between first infection and clinical manifestation [Reference Ammann and Eckert1]. Untreated, this disease remains fatal [Reference Kern, Kratzer and Reuter4, Reference Kern, Kratzer and Reuter5]. The incidence of the disease varies in the endemic regions of Central Europe between 0·02 and 1·4/100 000 persons [Reference Eckert and Deplazes6, Reference Gottstein7]. At present, only three studies have assessed prevalence using both serology and ultrasound; the remaining prevalence data are based solely on serological studies [Reference Bartholomot8–Reference Romig10].
Earlier prevalence studies have used Em2-, EgHF-, EmP-, Emc- and Emf-ELISAs for screening larger populations [Reference Craig9–Reference Gottstein13]. Currently, reports in the literature describe a total of 14 different enzyme-linked immunosorbent assays (ELISAs) (Emc-, Emf-, Em2-, Em2plus-, EgHF-, EmP-, Em10-, Em13-, Em16-, Em18-, Em70-, Em90-, EmAP- and EmII/3-10-ELISA) that have been used to diagnose AE [Reference Craig9–Reference Zhang, Li and McLanus19].
Previous data on the change in E. multilocularis antibody concentrations in AE over time are available only from a rural population in southern Germany [Reference Romig10, Reference Jensen20]. In this population, follow-up revealed no increase in disease progression or significant change in serological antibody concentrations. Data for an urban population have not yet been published. In November, 2002, a cross-sectional survey drawn from an urban population of Leutkirch, a town in southwestern Germany, using crude Emc- and Emf-ELISAs demonstrated that 95 individuals, or 3·9% of those tested, were seropositive to at least one of the two crude antigen ELISAs used [Reference Haenle21]. No clinical or ultrasonographic signs of AE were found in any of these subjects.
The objective of the present study was to re-examine these seropositive subjects and to assess the reliability of Emc- and Emf-ELISAs in predicting AE compared with the specific Em2plus-ELISA and diagnostic ultrasound screening.
METHODS
Follow-up study population
At the time of the initial survey in 2002, a total of 95 study participants tested positive with at least one of two raw antigen ELISAs, with 16 probands positive for Emc and 79 for Emf. Emc antigen was prepared from E. multilocularis metacestode tissue grown intraperitoneally in a laboratory strain of common voles as described previously by Gottstein et al. [Reference Gottstein22]. Emf antigen was vesicular fluid of E. multilocularis metacestodes grown in vitro as previously described by Hemphill & Gottstein [Reference Hemphill and Gottstein23]. All seropositive subjects identified during the initial EMIL study received a written invitation 10 months after completion of the screening to attend a follow-up appointment to include blood sampling and ultrasound examination. The follow-up examinations were conducted between November 2003 and February 2004 at the University Hospital Ulm Medical Centre.
Subjects who had not responded to the written invitation within 2 months were contacted by phone and again invited to a follow-up appointment. The most frequent reason stated for declining the follow-up appointment was the long distance to Ulm. Logistical and financial resources did not allow re-examination of the study participants at the original study site. Despite written invitation and telephone reminders, only 44 of the initial 95 seropositive subjects (46·3%) agreed to participate in the follow-up study appointment. Of these 44 subjects, 25 were female (average age 40·6 years, range 13–65 years) and 19 were male (average age 37·3 years, range 13–65 years). Only 31 subjects underwent both blood sampling and ultrasound examination. Twelve subjects underwent only ultrasound examination, which, in four cases, was performed at the University Hospital Ulm, and in the remaining eight cases at the practices of their respective primary-care physicians.
The study was conducted in accordance with the principles of the Helsinki Declaration and the GCP (Good Clinical Practice) recommendations. It was approved by the ethics commission of the Landesärztekammer Baden-Württemberg [Reference Haenle21].
Serological testing
Initial investigations in 2002
In the initial survey, 10 ml of whole blood was obtained from each subject for serological testing (Emc- and Emf-ELISA). After sedimentation at 7000–8000 g for 10 min, samples were stored at −70°C until final processing. Samples were tested for antibodies to E. multilocularis using an Emf-ELISA and Emc-ELISA (metacestode production in vivo in Meriones unguiculatus or metacestode in vitro culture according to Hemphill & Gottstein [Reference Hemphill and Gottstein23]). Antigens were coated onto microtitre plates (Nunc MaxiSorp, Nunc GmbH & Co., Wiesbaten, Germany) with a protein concentration of 3 mg/ml of Emf and Emc antigen. The participants' sera were investigated by ELISA at a dilution of 1:200. Anti-human IgG horseradish peroxidase was applied as conjugate at a standard dilution of 1:10 000 as indicated by the manufacturer (Dako, Deutschland GmbH, Hamburg, Germany). Tetramethylbenzidine dihydrochloride (TMB) served as substrate, and absorbance values were read at 450 nm.
Serological findings were considered as positive if the index reached a level of mean optical density plus 3 standard deviations, corresponding to >72 for Emc-ELISA and >110·3 for Emf-ELISA. All other findings were considered negative. All samples were tested in the laboratory of the State Health Department, Stuttgart, Germany.
Follow-up in 2004
As part of the follow-up examination conducted in 2003–2004, 10 ml of whole blood were obtained from 31 subjects for serological testing (Emc- and Emf-ELISA). Blood samples were processed and stored as described above.
Repeat serological testing in 2006
In 2006, in order to exclude methodologically based changes in concentrations and to enhance comparability of test results, we re-tested all sera obtained at the initial survey in 2002 and at the follow-up examinations in 2003–2004. For Emc- and Emf-ELISA, the methods used were identical to those used in 2002. All blood samples were tested in the laboratory of the State Health Department, Stuttgart, Germany.
In addition, all samples from the initial survey in 2002 and from the follow-up examinations in 2003–2004 were tested using Em2plus-ELISA (DPC Biermann GmbH, Bad Nauheim, Germany). The sensitivity of this commercially available test is reported as 97% [Reference Gottstein14]. The E. multilocularis Em2plus-ELISA is a solid-phase ELISA using microtitre plates for detecting serum IgG antibodies to E. multilocularis. The microtitre strips included with the test kit are coated with E. multilocularis Em2plus antigen. This is a mixed antigen comprised of an affinity-purified Em2 antigen and a recombinant II/3–10 antigen from E. multilocularis. The absorbance values obtained by the EM2plus-ELISA were measured at 405 nm. The Em2plus-ELISA testing was conducted in the Department of Medical Microbiology and Hygiene of the University Hospital Ulm.
Ultrasound examination
Ultrasound follow-up examinations were performed at the University Hospital Ulm using a HDI 5000 ultrasound scanner (ATL Ultrasound, Philips Medical Systems, Bothell, WA, USA) of the same type as used at the initial survey in Leutkirch in 2002 [Reference Haenle21]. Ultrasound follow-up examinations outside the University Hospital were done by primary-care physicians. All of the physicians had a licence to perform ultrasound examinations from the Medical Council. On examination, the liver was assessed for evidence of infection with E. multilocularis; the examination also included assessment of the kidneys, gallbladder, biliary tract and spleen.
RESULTS
In the initial survey of the EMIL study in 2002, 95/2445 subjects from an urban population in southwest Germany were found to be positive on at least one of two raw antigen ELISAs (Emc-ELISA, n=16; Emf-ELISA, n=79) for evidence of infection with E. multilocularis. The following data are results of the re-testing in 2006, conducted under identical laboratory conditions, of cryopreserved sera of 31 subjects who participated in the initial (2002) and follow-up examinations (2003–2004).
Initial sera (2002)
Of the 31 sera found to be positive (Emc and Emf) at the initial survey in 2002, only 26 tested positive at re-testing conducted in 2006. Of these, eight tested positive with Emc-ELISA and 13 with Emf-ELISA, while three remained positive with both tests. Five initially positive sera tested negative at re-testing despite application of identical testing procedures. All sera initially positive in 2002 tested negative by Em2plus-ELISA (see Table 1).
Table 1. Results of Emc- and Emf-ELISA in 2002 and at follow-up in 2004
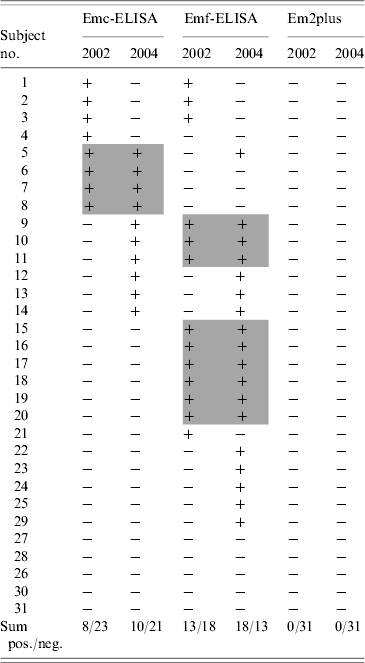
Emc-ELISA, Antigen obtained from metacestodes; Emf-ELISA, antigen obtained from vesicular tissue; +, positive; −, negative.
Grey shading indicates sera which have been positive for Emc-ELISA and Emf-ELISA in 2002 and 2004.
Follow-up sera (2004)
Of the 31 sera obtained at the follow-up examinations in 2004, only 21 were positive when tested again in 2006. Seven of the 21 sera were positive by both Emc- and Emf-ELISA, while three sera were positive only by Emc-ELISA and 11 only by Emf-ELISA. All 21 sera testing positive in the follow-up examinations in 2004 tested negative with the Em2plus-ELISA (see Table 1).
Comparison of the 2002 and 2004 sera
A comparison of Emc-ELISA concentration results obtained in 2002 and 2004 showed that the number of subjects seropositive by this assay increased from eight to ten. Among the total of 14 subjects positive by Emc-ELISA in 2002 or 2004, follow-up testing in 2004 revealed agreement with the 2002 results in only four cases. A comparison of Emf-ELISA concentration results obtained in 2002 and 2004 showed that the number of subjects seropositive by this assay increased from 13 to 18. Among the total of 22 subjects positive by Emf-ELISA in 2002 or 2004, follow-up testing in 2004 revealed agreement with the 2002 results in nine cases (Table 1).
Ultrasound
Study subjects undergoing follow-up ultrasound examinations at the University Hospital Ulm or by their respective primary-care physician did not identify any findings specific for E. multilocularis disease. In 30 subjects, the findings of the diagnostic ultrasound were within normal limits and appropriate for their respective ages. Coincidental findings included hepatic steatosis in five cases, liver cysts in two cases, a haemangioma in one case, gallbladder stones in two cases, and gallbladder polyps in four cases. None of the findings at follow-up ultrasound had immediate diagnostic or therapeutic relevance.
DISCUSSION
Few studies have used serology and diagnostic ultrasound to assess the prevalence or seroprevalence of E. multilocularis in follow-up [Reference Romig10, Reference Jensen20]. It is still unclear to what extent seropositive individuals may be at risk of subsequently developing manifest AE. Because seroprevalence rates up to 20% can be found in highly endemic areas [Reference Craig9, Reference Romig10], understanding the relationship between seropositivity without detectable lesions on ultrasound examination and future disease is of great importance in developing follow-up strategies to reduce morbidity and mortality associated with AE.
The findings of the present study show significant changes in Emc- and Emf-ELISA antibody concentrations over time using these non-specific assays. The results from antibody concentration determinations performed in 2006 did not reproduce the initial concentrations from 2002 despite using identical test procedures. Agreement between the two test methods was observed in only three subjects in 2002 and in only seven subjects in 2004. For Emc-ELISA, comparison of 2002 and 2004 results revealed agreement in only four subjects, while, for Emf-ELISA, there was agreement in only nine cases. The inconsistency between 2002 and 2004 measurement results must, therefore, be seen as a basic method-related problem with this screening test and underscores the poor reliability of these methods. The highly specific Em2plus ELISA was negative for all sera from both the 2002 and 2004 series, consistent with both the results of the ultrasound examination and the reported high specificity of this test [Reference Gottstein14].
A study conducted in 1996 in the southwestern German town of Römerstein [Reference Romig10] used the same serological tests as were used in our study. Three years after completion of the initial Römerstein study, 36/47 (76·6%) seropositive patients participated in a follow-up study [Reference Jensen20]. At follow-up, ultrasound examination did not demonstrate findings suggestive of E. multilocularis lesions in any study subject.
The studies described above of patients with persistently elevated antibody concentrations to E. multilocularis antigens without evidence of typical liver lesions on ultrasound may represent latent AE, which, with its long incubation period of 10–15 years [Reference Ammann and Eckert1] has not yet become manifest. An alternate explanation for persistently positive serologies in individuals without evidence of active disease may be immunity among persons residing in areas highly endemic for E. multilocularis [Reference Lanier24]. According to Gottstein and colleagues [Reference Gottstein7, Reference Gottstein and Felleisen25], only about 10–30% of persons undergoing seroconversion following exposure to E. multilocularis subsequently develop AE [Reference Gottstein and Felleisen25]. In addition, there may be individuals who, despite a high risk of exposure to E. multilocularis, fail to mount an immunological response to parasitic antigens. Finally, E. multilocularis seropositivity in clinically healthy subjects might be due to cross-reactivity of the raw antigen ELISAs, as has been described previously [Reference Gottstein14].
Vuitton describes four possible situations in which an individual may develop antibodies to E. multilocularis. First, a person may already exhibit clinically manifest AE; second, the person may suffer from latent disease that has not yet become clinically apparent; third, there may be extinct and calcified lesions in the liver; fourth, there may be no detectable lesions whatsoever [Reference Vuitton26]. A study conducted in Alaska reported cases of AE in which the parasites have spontaneously died out: in these cases, Em2-ELISA remained persistently positive [Reference Stehr-Green27].
There are also reports in the literature of protective factors against infection with E. multilocularis. The HLA allele HLA-DRB1*11 has been associated with protection against development of AE [Reference Eiermann28]. In the same study, other HLA alleles such as HLA-DQB1*02 appeared to be associated with a progressive disease course. In another study [Reference Godot29], it was shown that patients with the HLA DR3+ DQ2+ haplotype secrete higher levels of TH2 cytokine, which promotes disease progression.
The seropositive subjects in the present study using unspecific ELISAs such as as Emf and Emc seem not to suffer from the early stages of AE. Seropositivity to these antigens may simply be a cross-reaction [Reference Muller30]. The results of our study do not allow us to postulate subjects' possible contact with E. multilocularis antibody or to assume a certain degree of immunity to the parasite, especially since the more specific Em2plus-ELISA and ultrasound remained negative at both the initial and follow-up surveys.
A limitation of the study might be the long period of sample storage. To our knowledge there exists no study, which systematically investigated the stability of antibody concentrations in blood sera over years. However the experience of the Baden-Württemberg State Health Office suggests a high quality of samples stored at −70°C over many years.
Follow-up of subjects seropositive by Emc- or Emf-ELISA screening surveys and without sonographic evidence of disease does not appear to be justified at this time.
APPENDIX. Members of the EMIL Study Group
G. Adler, A. Armsen, H.-M. Banzhaf, M. Bauerdick, U. Bertling, B. O. Boehm, B. O. Brandner, S. O. Brockmann, M. Deckert, C. Dingler, S. Eggink, M. Fuchs, W. Gaus, H. Goussis, A. Gruenert, M. M. Haenle, W. Hampl, C. Haug, B. Hay, M.-L. Huetter, A. Imhof, P. Kern, P. Kimmig, A. Kirch, D. Klass, W. Koenig, W. Kratzer, M. Kron, B. Manfras, K. Meitinger, T. Mertens, R. Oehme, G. Pfaff, I. Piechotowski, S. Reuter, T. Romig, A. F. A. von Schmiesing, G. Steinbach, M. Tourbier, A. Voegtle, T. Walcher, S. Wolff.
ACKNOWLEDGEMENTS
This study was initiated by the government of the State of Baden-Württemberg, Germany. Financial support was provided by the Baden-Württemberg State Health Office (District Government Stuttgart, Germany) and the Regional Health Office of Ravensburg, Germany. Further support was provided by the City of Leutkirch, Germany. Tubes for blood sampling and part of the laboratory testing tubes were supplied by Sarstedt AG & Co., Nümbrecht, Germany. The authors express their special gratitude to Mr Walter Feucht of the firm of ULDO-Backmittel GmbH (Neu-Ulm, Germany) for his generous support of this study.
DECLARATION OF INTEREST
None.





