INTRODUCTION
Bovine tuberculosis (bTB) caused by Mycobacterium bovis infection is an important infection of cattle and can also infect a wide range of domestic and wild animals as well as humans [Reference O'Reilly and Daborn1]. Cattle act as the maintenance hosts in many countries and the infection has proved extremely difficult to eradicate in domestic species once it has become established in a wildlife reservoir [Reference Palmer2]. The incidence and epidemiology of bTB varies widely both internationally and locally [Reference Abernethy3–Reference Cousins and Roberts5]. Much of Europe is officially bTB free but the infection is endemic in cattle in parts of England, Wales, Ireland and Spain and other parts of Europe, which are dissimilar in terms of environmental features and wildlife reservoirs [Reference Ramirez-Villaescusa6, Reference Allepuz7]. Transmission of M. bovis is influenced by many factors including the intrinsic characteristics of the bacterium and potential host, the persistence of the bacterium in different environments, the probability of exposure of infected animals, e.g. cattle or wildlife vectors and/or fomites and the effectiveness of control strategies such as removal of infected animals.
Assessment of risk is dependent on unbiased measurement of factors associated with transmission between the bacterium and host and accurate classification of the infection status of animals and herds. A risk factor is defined as a characteristic, that in its presence increases the likelihood of the detection of infection, and they may be causal or non-causal [Reference Thrusfield8]. Many risk factors are inter-related, e.g. each animal production system has its own distribution of sexes, breeds and age groups, which contribute to the exposure profile of each herd. Diagnostic tests for bTB range from those used in live cattle [e.g. tuberculin skin test (TST) and blood tests for interferon-gamma (IFN-γ) or antibodies] to post-mortem inspection confirmed with culture for M. bovis. Intrinsic test performance varies with the animal's immunological profile, disease infection stage and many other factors. For example, increasing the frequency of skin tests, a feature of intensified surveillance and control strategies for bTB, can modulate cell-mediated immunity and depress the response to subsequent skin tests [Reference Coad9, Reference Thom10].
The relative importance of different risk factors for M. bovis transmission will vary with the background prevalence and magnitude of other risks. An understanding of risks and how they may interact is therefore necessary to understanding the epidemiology of the disease and the design of bTB eradication and control policies and forms the focus of this review.
METHODS AND RESULTS
A series of search strings were developed to identify relevant papers from CAB Abstracts, Web of Science, Medline and Current Contents electronic databases (see Supplementary material for the specific search strings). Two sets of searches were performed, the first deliberately excluding Mycobacterium avian paratuberculosis (MAP) and the second, specifically including it as a risk factor. The results of these were then combined. The search period was not limited historically and initially ended on 14 March 2013. Abstracts to 3738 non-duplicate references identified were reviewed by three reviewers to determine whether the entire article was likely to contain relevant information. Twenty-eight abstracts were reviewed by two reviewers to detect any perceived inconsistencies in selection. Inconsistencies were resolved through discussion, which then informed abstract selection. All publications that specifically investigated the risk of infection to cattle, or where that risk could be inferred, were included and the full text was requested. Papers in German, Spanish, Italian and French that were selected for further review were translated into English. Entire texts for selected papers were then obtained and assigned to one of ten full-text reviewers who each had a specific risk factor category as follows: animal-level, herd-level, environment and landscape features, herd history including previous testing, wildlife factors, and other risk factors. Reviewers were free to add further papers that had been omitted during the electronic search. The search was updated to 6 February 2015 using the original search criteria and a further 517 references identified, of which 81 were considered potentially relevant by one of the original reviewers.
The search was not limited geographically, but during the review emphasis was placed on references that could improve understanding of the Great Britain (GB) epidemiological situation. Methodology and reporting was consistent with PRISMA (http://www.prisma-statement.org) where possible with respect to the introduction, eligibility criteria, information sources, search and discussion, and as far as the qualitative assessment of the publication allowed.
ANIMAL-LEVEL RISK FACTORS
Genetics
Significant variation in heritability of susceptibility to bTB has been reported in studies of dairy cattle, mainly Holstein Friesian pure bred or crosses, in GB [Reference Brotherstone11] and in Ireland [Reference Bermingham12]. In these population-based studies, around 16% and 18% of the variance in bTB resistance, measured by responsiveness to the single intradermal comparative cervical tuberculin (SICCT) test and post-mortem evidence of infection, respectively, was estimated to be heritable [Reference Bermingham13]. There has been less investigation of heritability of resistance in beef herds. However, a study in Ireland has estimated heritability of susceptibility to bTB of around 13% in beef herds and also reported variation in susceptibility across different beef breeds [Reference Richardson14]. These large studies, using surveillance data from thousands of cattle, may underestimate the contribution of heritability due to misclassification of the resistant phenotype because of imperfect accuracy of diagnostic tests and variation in level of exposure to M. bovis [Reference Allen15]. Greater variability in the estimates of heritability of susceptibility to bTB has been observed in high- compared to low-infection prevalence herds [Reference Richardson14].
Resistance to bTB is likely to be multifactorial and polygenic [Reference Morris16]. In addition to heritability studies using phenotype data, candidate and whole genome approaches are being taken [Reference Allen15]. These approaches have indicated that resistance can be conferred by a single nucleotide polymorphism. Both breeding strategies and genomic selection are likely to have utility in bTB control [Reference Driscoll17–Reference Song19]. Breeding for performance traits has led to large increases in milk outputs from dairy cows. Until recently the latter was accompanied with a reduction in fertility [Reference Butler20]. No association between susceptibility to M. bovis infection and economically important traits has been measured to date [Reference Bermingham21].
Breed
The earliest evidence for differences in resistance to bTB infection by breed originates from the 1920s and 1930s [Reference Allen15, Reference Vordermeier22]. Evidence mainly originates from Africa and strongly suggests that native cattle such as zebu, found in pastoral environments, are more resistant to bTB than introduced European cattle [Reference Vordermeier22–Reference Ameni and Erkihun26]. A weakness of some comparisons of bTB prevalence between breeds is the reliance on an ante-mortem test which may be less specific than evidence from post-mortem examination. There has been comparatively far less evaluation of the differences in the susceptibility to infection between European breeds. A study in India showed a threefold higher prevalence of SICCT test reactors in Jersey cattle compared to Holstein Friesian [Reference Thakar27] but differences in exposure to M. bovis between breeds require exploration.
The UK Bovine HapMap Consortium has illustrated that greater diversity exists in allele frequencies between cattle breeds than within breeds [28]. Greater variability in resistance might be anticipated with beef cattle than with cattle in the modern dairy sector since the latter are more homogeneous in terms of breed.
Sex
Differences in bTB prevalence by sex are confounded by differences in the production environment and husbandry. Contact rates are higher for many dairy cattle. Additionally, milk-producing dairy cattle and suckler cows tend to have longer lives than beef cattle, increasing the lifetime probability of exposure to M. bovis [Reference Alvarez29, Reference Bell30]. Incidence of bTB infection in dairy cattle, aged ⩾2 years, was 40% higher than beef cattle, although incidences in very young cattle (0–1 years) were similar [Reference Brooks-Pollock31]. Most studies report a higher prevalence or incidence of bTB in female cattle compared to males [Reference Brooks-Pollock31–Reference Yacob, Basu and Guesh37], but not all [Reference Kazwala38], and none comprehensively controlled for differences in infection pressure, production environment and husbandry. At this time, an intrinsic difference in susceptibility to bTB between male and female cattle has not been demonstrated.
Reproductive status and milk yield
Few studies have examined the effect of reproductive status on susceptibility to bTB infection, possibly because of the difficulty in separating out gestation and lactation effects. The dairy cow experiences large hormonal shifts and stresses throughout production and it seems likely that these will affect both her response to diagnostic tests that measure immunological factors and susceptibility to infection. In contrast to susceptibility to bTB infection, cows in the weeks before and after calving tend to have weaker reactions to the TST, but the IFN-γ is less affected [Reference Wood and Jones39]. Evidence is primarily found in studies outside of Europe and in the absence of stringent control programs where animals may experience advanced clinical disease. In Bangladesh, consumption of infected milk is also a potential (pseudo-vertical) transmission route for infection between cows and calves and a higher prevalence of bTB was observed in lactating cows compared to pregnant cows [Reference Rahman and Samad40]. In Argentina, calves fed raw milk from a herd with endemic bTB were more likely to test positive (caudal fold test) than calves fed replacement milk substitute [Reference Garro41]. However, a large cohort study in Northern Ireland found no evidence of an increased risk of bTB in the progeny of TB-confirmed dams compared to those born to non-reactors in the same herd [risk ratio 1·2, 95% confidence interval (CI) 0·8–1·79] [Reference Menzies42].
Age
Virtually all studies reviewed reported that the prevalence of bTB infection increased with age [Reference Cadmus23, Reference Thakar27, Reference Singh34, Reference Bernard43–Reference Moiane48]. The relationship between bTB infection and age is generally shown as monotonic or linear although a U-shaped relationship has also been reported [Reference Cadmus23, Reference Wolfe49]. Modelling bTB surveillance data from GB showed that age-specific incidence increased monotonically to 24–36 months, followed by a levelling-off, with cattle aged between 12 and 36 months experiencing the highest rates of infection [Reference Brooks-Pollock31]. The most likely explanation for a positive correlation between bTB infection risk and longevity is a higher probability of contact and/or prolonged exposure to other infected cattle, wildlife or environmental contamination; infected cattle aged >36 months are possibly more responsive to the skin test [Reference Brooks-Pollock31] and therefore more likely to be detected in surveillance tests. A review of age effects in Tanzania, Zambia, Chad and Ireland also concluded that the positive association with increasing prevalence of bTB is a result of cumulative exposure [Reference Humblet, Boschiroli and Saegerman50]. Other possibilities include decline in resistance with age [Reference O'Reilly and Daborn1]. The influence of latency on detection and transmission of bTB in cattle is not known, although it is recognised as an important influence on disease prevalence and transmission in human infection with M. tuberculosis [Reference Pollock and Neill51].
Nutritional status and body condition
Poor nutrition can suppress the cellular immunity response and increase susceptibility to infectious diseases [Reference Field and Johnson52–Reference van Crevel54]. Responsiveness to the SICCT test was positively correlated with body condition score and fat production in lactating Holstein-Friesian dairy cows in GB, which implies some association between susceptibility or at least responsiveness to the diagnostic test [Reference Bermingham21]. A cross-sectional study of cattle from herds with bTB infection found that significantly lower levels of the dietary selenium-requiring enzyme glutathione peroxidase were associated with a higher prevalence of post-mortem confirmed infection [Reference Downs55]. However, two Irish studies comparing animals with energy-restricted and unrestricted diets did not detect significant differences in bTB infection [Reference Doherty56, Reference Costello57]. Reports from Ethiopia are similarly mixed, with one study reporting a higher prevalence of bTB in animals with lower body condition scores [Reference Biratu47] while another found no differences [Reference Gumi44]. Future research needs to distinguish nutritional status/dietary factors and body condition from the immunological consequences of infection and the possible confounding effects of breed and other factors.
Behavioural factors
Contact networks between and within wild and domestic animals are heterogeneous [Reference White58, Reference Sauter and Morris59]. Cattle are inquisitive towards new objects and calves and some adult cattle are particularly so [Reference MacKay and Wood-Gush60]. A study using proximity data loggers identified individual adult cattle with higher levels of contact both with other cattle in the herd and with badgers [Reference Bohm, Hutchings and White61]. In a New Zealand study, 86% of the tuberculin test-positive cattle were among the most dominant fifth in the herd, and in four of five herds, the dominant animals investigated a sedated possum (the principal wildlife vector of bTB) most actively [Reference Sauter and Morris62]. The differing contact behaviour of cattle towards live and inanimate objects implies that the individual risks of exposure to infection are heterogeneous.
Concurrent infection
Parasitic helminth infection status has been shown to be negatively correlated with bTB infection status in bovids [Reference Flynn63–Reference Ezenwa65]. Evidence suggests that infection with the helminth Fasciola hepatica may bias the immunological response towards a Th2 (eosinophilic) type at the expense of the Th1 (cell-mediated) immune response that influences the ability to control M. bovis [Reference Ezenwa65]. Concurrent infection with or vaccination against MAP can influence the sensitivity and specificity of diagnostic tests for bTB [Reference de la Rua-Domenech66, Reference Morrison67] (see below for further discussion). Outbreaks of bTB in two herds with a concurrent bovine viral diarrhoea (BVD) infection have been described anecdotally [Reference Watson68]. Compared to calves with M. bovis infection alone, calves co-infected with BVD and M. bovis show non-significantly increased bacterial shedding in nasal secretions, in experimental infections [Reference Kao69]. Further studies are needed, however, to evaluate whether there is an association between the two diseases.
HERD-LEVEL RISK FACTORS
Many factors describing the characteristics and management of herds have been associated with infection risk, but the direction of findings is inconsistent, even between studies conducted using a similar sampling frame [Reference Johnston70, Reference Johnston71], possibly due to difficulty in characterising management factors accurately. A number of observational studies relating to herd-level risk factors within the UK and Ireland in specific circumstances have been well reviewed recently [Reference Godfray72–Reference Vial75]. Rather than add to these, we have supplemented these with information from other countries that may inform the situation in GB.
Herd size
Herd size is the most frequently identified risk factor for bTB incidents (herds that are found to have at least one infected animal). Dynamic modelling has shown that, adjusting for test performance, the increased probability of between-animal contacts in larger herds could increase within-herd transmission [Reference Conlan76]. Most studies, but not all [Reference Omer77–Reference Garro79], conclude that the risk of infection detected in a herd increases with herd size; this holds true across a broad geographical and prevalence range (Table 1). Herd size may be a proxy for other factors, such as replacement policy, farm acreage, number of premises and neighbouring herds; each of which may be independently associated with the risk of a bTB incident. Additionally, most studies have not adjusted for the imperfect specificity of diagnostic tests for bTB which may affect the strength of association since the number of animals that are false positives will also increase with herd size. However, increased herd size could also result in increased risk of disease persistence.
Table 1. Risk factors identified in the literature according to theme, country in which the study was conducted and case definition

SICCT, Single intradermal comparative cervical tuberculin test; PMC, post-mortem confirmed; Rct, recurrent TB incident; Pst; persistent TB incident.
Herd type
Dairy herds are generally considered to be more at risk of infection than beef herds because of longer life expectancy of the former and management practices that increase the risk of contact with other cattle [Reference Brooks-Pollock31, Reference Humblet, Boschiroli and Saegerman50, Reference Goodchild and Clifton-Hadley80]. Relatively few studies have considered herd type and of those, even fewer studies differentiate between beef fattening/finishing herds and beef breeding/suckler herds. The relative risk to beef cattle may be greater in areas where there is a local wildlife reservoir because beef cattle more frequently use rough or dispersed grazing. Direct contact between cattle and badgers at pasture is relatively rare [Reference O'Mahony81], although indirect contact may be more common. In Italy, enterprises consisting of both dairy and beef animals, presented a greater risk [odds ratio (OR) 4·92, 95% CI 1·26–19·19] than exclusively dairy enterprises, attributed to differences in purchasing policy [Reference Marangon78]. Dairy herds experienced a recurrent bTB incident sooner than non-dairy herds in Northern Ireland, even after controlling for herd size [Reference Doyle82], but a similar study in Ireland showed that dairy animals had a lower risk of a future restriction [Reference Gallagher83]. GB surveillance data indicate that the relationship between bTB incidence rate and herd type may be a result of the larger sizes of dairy herds and their location [84].
Dairy reactors to field tests for bTB appear to be less likely than non-dairy reactors to have post-mortem evidence of infection such as culture, histological evidence or macroscopic lesions typical of bTB PMC than non-dairy [Reference Downs55, 84–Reference O'Hagan86]. In Northern Ireland the adjusted odds ratio for visible lesions in non-dairy reactors detected during routine surveillance was reported to be twice that in dairy [Reference O'Hagan86]. The reason for the differences in post-mortem evidence of infection confirmation between dairy and beef production classes exposed to bTB is currently unknown [Reference Downs87].
Total farm area, fragmentation and neighbouring herds
Many authors identified the importance of contact between adjacent herds as a risk factor. This can be expressed in terms of lengths of common boundary, fragmentation of holdings, or observed contact with cattle from contiguous farms [Reference Johnston70, Reference Griffin88, Reference Denny and Wilesmith89]. Farm size (acreage) has been associated with a higher probability of a bTB incident [Reference Mill90, Reference Vial, Johnston and Donnelly91], even after controlling for herd size [Reference Guta92] and it may be a proxy for increased risk of infection from contiguous premises or for increased wildlife contact. Operating over multiple premises has been associated with infection in GB [Reference Johnston71, Reference Mill90] although not in all studies [Reference Vial, Johnston and Donnelly91, Reference Reilly and Courtenay93].
Many studies have shown an increased risk of bTB spread associated with bTB in neighbouring or contiguous herds. In Ireland, 23–25% of incidents were attributed to spread from herds on adjacent farms [Reference Griffin and Dolan94] and herds with a neighbouring infected herd were almost four times as likely to sustain a bTB incident themselves [Reference Griffin95]. In Northern Ireland, ~40% of breakdowns were attributed to the presence of a neighbouring herd with a breakdown [Reference Denny and Wilesmith89]. Contact with an infected neighbouring cattle herd was associated with persistent incidents (lasting more than 5 years) in Spain [Reference Guta92] and was associated with a more than twofold increase in the odds of a bTB incident in high incidence areas of England and Wales [Reference Johnston70]. In Ireland, it was estimated that being within 1 km of higher animal incidence in the previous 2 years accounted for 35% of bTB incidents [Reference White96]. However, distinguishing between spread from one herd to another and sharing a common source of infection is difficult where there is a significant reservoir of infection in wildlife.
Cattle movements
A large number of studies have considered the risk of introducing stock in general and in specific scenarios, such as restocking after foot-and-mouth disease in 2001 in UK [Reference Ramirez-Villaescusa6, Reference Carrique-Mas, Medley and Green97, Reference Ramirez-Villaescusa98] (Table 2) and the influence of trading in England and Ireland has been well reviewed [Reference Godfray72, Reference Skuce, Allen and McDowell73, Reference Vial75]. All report an association between restocking and subsequent increased risk of infection [Reference Johnston71, Reference Marangon78, Reference Gilbert99–Reference Ward, Tolhurst and Delahay101]. Molecular epidemiology is used increasingly to confirm sources of incidents [Reference Gopal102, Reference Humblet103]. The reported percentage of bTB incidents attributed to cattle movements in Ireland, where disease is spread throughout the country, varies from 6% to 15% [Reference Humblet103–Reference Griffin105]. In GB, where disease is clustered in the West and South-West Wales, the proportion of infection attributable to purchased infection is much higher in lower incidence areas. An investigation of 31 incidents in a low bTB incidence area of England found that all but one were most likely due to purchased animals [Reference Gopal102]. Prior to legislation requiring the pre- and post-movement testing of cattle imported into Scotland from regions with a high bTB incidence, Scottish farms importing cattle from these regions were almost four times as likely to experience a breakdown than farms that did not. Following the legislation change (2006–2009), this risk, while still significant, had reduced substantially (OR 2·5, 95% CI 1·03–6·27) [Reference Gates, Volkova and Woolhouse106].
Table 2. Studies investigation an association between introduction of stock and bTB incident

OR, Odds ratio; CI, confidence interval; RBCT, Randomized Badger Culling Trial; SICCT, single intradermal comparative cervical tuberculin test.
Markets and agricultural shows
The evidence for a role of markets in bTB disease transmission is inconsistent. Moving animals into herds from markets has been identified as a significant risk factor in GB [Reference Ramirez-Villaescusa6, Reference Johnston71]. One analysis found that herd owners buying cattle from markets had a lower risk of bTB infection than those that did not but this only applied to one region and it was not significant when herds that had been under restrictions, and so could not purchase, prior to the study were excluded [Reference Johnston70]. The largest investigation found no relationship between movement or purchasing via markets and bTB in 1544 lesioned, TST reactors compared to matched controls in Northern Ireland [Reference Abernethy, Pfeiffer and Neill107]. The evidence for a role for agricultural shows in transmission is anecdotal. A descriptive account of an outbreak in the 1970s found that 51 of 56 cattle from 42 herds reacted to the skin test 80 days after being at the same agricultural show [Reference Steger108].
Farm management
There is evidence that ‘good’ management and hygiene practices may reduce the risk of M. bovis transmission, particularly in developing countries [Reference Elias109–Reference Khan and Khan113] but also in Europe [Reference Christiansen and Clifton-Hadley114, Reference Cowie115]. In Spain, the communal use of equipment (cattle race) between herds was positively associated with a positive TB test [Reference Cowie115]. In GB, poor dairy herd management such as; farm buildings and feed stores being accessible to badgers, allowing grazing at field margins and grazing in fields with badger activity were associated with an increased risk of bTB [Reference Christiansen and Clifton-Hadley114].
Intensive management practices that increase cattle to cattle contact within a herd are associated with an increased risk of bTB incidents [Reference Winkler and Mathews116]. Case-control studies have found dairy herds kept in cubicle housing [Reference Griffin95] and covered yards [Reference Johnston71] have a substantially increased risk of bTB. This type of housing may be a proxy for the effects of greater stress or reflect a more intensive management system that increases cattle contact rates. This is supported by studies in GB that found the presence of a loafing yard or paddock adjoining cattle housing [Reference Christiansen and Clifton-Hadley114] and not providing housing for cattle [Reference Vial75] reduced the risk of bTB. The use of barns in which cattle can move freely was associated with a positive SICCT test in Spain; the aggregation of cattle within the barn increasing both direct and indirect contacts [Reference Cowie117].
Rough grazing in Ireland [Reference Griffin95], set stocking in England [Reference Johnston70] [Reference Christiansen and Clifton-Hadley114] and grazing cattle on sown or ley pasture [Reference Christiansen and Clifton-Hadley114] have all been associated with an increased risk for bTB in multivariate analyses controlling for herd size. Feeding from the ground either at pasture or during housing [Reference Cowie115], storing silage in clamps [Reference Reilly and Courtenay93] and feeding green foods/kale or barley have been associated with an increased risk of a bTB incident [Reference Christiansen and Clifton-Hadley114]. These findings could be potentially attributed to increased contact with wildlife. Silage, in particular maize silage [Reference Lanszki118] is attractive to badgers, and was found to be associated with badgers entering farm buildings in one study [Reference Garnett, Delahay and Roper119], but visits to silage clamps were rare in another larger scale study [120]. The storage and feeding of silage from clamps is associated with persistent breakdowns [Reference Reilly and Courtenay93] potentially related to the clamps being more accessible to badgers. Feeding hay rather than silage/concentrates has been reported as protective [Reference Reilly and Courtenay93, Reference Griffin95, Reference Griffin and Hahesy121], and a case-control study found herds fed silage had a greater risk of a breakdown [Reference Winkler and Mathews116]. In one study, all farms that experienced a persistent breakdown were fed grass silage, although 85% of the TB-free farms also did so [Reference Reilly and Courtenay93].
Three studies have found that nutritional supplementation lowers the herd-level risk of bTB. Farms with areas of rough grazing that did not supply mineral licks were up to 30 times as likely to be infected with bTB, although with wide 95% CIs (1·46–594·4) suggesting it should be interpreted with caution [Reference Griffin95]. Two further studies in England support the finding, however, as the provision of protein supplement blocks [Reference Christiansen and Clifton-Hadley114] and vitamin and mineral licks [Reference Ramirez-Villaescusa6] were protective.
There is some evidence, although none from the UK and Ireland, of an increased risk of transmission of M. bovis infection to calves fed raw milk or colostrum. However the evidence as to its contribution to bTB risk within the context of an ongoing control program is weak. A case-control study in Argentina found no increased risk in herds feeding raw milk, but did find a higher risk in herds where weaning had been delayed for 4 days, which could be attributed to either drinking infected colostrum or increased exposure to an infected dam [Reference Garro79]. In Spain, bTB incidents in herds where calves were fed replacement milk from test-positive dams were more likely to be persistent but this verged on significance [Reference Guta92].
Other domestic species
While M. bovis has been isolated from a range of non-bovine domestic species in GB [Reference Broughan122], they are not thought to represent a substantial risk to cattle. Few studies have directly examined the risk they pose and no clear trend has been identified [Reference Guta92, Reference Karolemeas123, Reference Karolemeas124]. In Spain, bTB incidents were almost four times more likely to be persistent if goats were present on the farm, although only a small number of farms were affected (N = 11) while incidents in herds that also farmed pigs were more likely to be resolved quickly [Reference Guta92]. Conversely, contact with any domestic species from a non-contiguous farm was associated with an increased risk of a prolonged incident in GB [Reference Karolemeas123].
bTB HISTORY AND TESTING
bTB history in the region and herd
Local geographic areas with a history of bTB have been consistently identified as being at significantly higher risk of future incidents than other areas [Reference White and Benhin100, Reference Humblet103, Reference Olea-Popelka125–Reference Dawson127]. The origins of recurrent infection are difficult to separate. There may be a failure to detect and remove all infected cattle or there may be repeated reinfection of the herd through exposure to one of several risk factors which might include infected cattle in contiguous herds, local movements and exposure to other wildlife or environmental sources of M. bovis. Evidence that reinfection can cause recurrent bTB in GB has come from studies of bTB incidents in herds after complete depopulation and restocking, ruling out infection persistence within the herd [Reference Carrique-Mas, Medley and Green97, Reference Green and Medley128]. In Ireland, herds depopulated for bTB control were no more likely to have a further bTB incident than herds that had been depopulated because of BSE control, but localized badger culling prior to depopulation had occurred in the bTB herds [Reference Good129].
The number of reactors or the duration of previous bTB incidents is also associated with future risk at a herd level [Reference Doyle82, Reference Gallagher83, Reference Karolemeas123, Reference Olea-Popelka125–Reference Dawson127, Reference Wolfe130] and also at an animal level [Reference Green131, Reference Berrian132]. Post-mortem evidence of infection in cattle in the original incident has been positively associated with a higher future incident risk in at least one study in Ireland [Reference Olea-Popelka125], although in others no such relationship has been found [Reference Doyle82, Reference Karolemeas123, Reference Abernethy, Alban and Kelly126, Reference Wolfe130]. Mathematical modelling of recurrence of bTB within a herd estimated that around 24–50% of recurrent incidents are attributable to persistence of infection in the herd, depending on the length of the assumed latent period [Reference Conlan76].
In some countries including GB, inconclusive reactors (IRs) are re-tested and, depending on the result, either remain in the herd or are culled. Follow up of IRs has found that they are more likely to have post-mortem evidence of infection than cattle that had a negative response to the skin test [Reference Clegg133]. Furthermore, IRs that re-tested negative and remained in the herd were almost four times more likely to be diagnosed with bTB at a later date [Reference Clegg134].
Persistence and test performance
Recurrence and infection persistence in a herd may indicate failure of testing, thereby allowing infected animals not only to remain in the population, but potentially to continue to spread infection. Several factors have been identified that influence test performance and hence possible failure to detect infection:
-
(1) Intrinsic test sensitivity. Diagnostic accuracy varies between different types of tests and tests currently approved for bTB in Europe are considered to have fairly moderate sensitivity and variable specificity [135]. Potency of the tuberculin (purified protein derivative; PPD) may also affect the sensitivity and specificity of tests that rely on this biological product such as TST and the IFN- γ blood test [Reference Monaghan136–Reference Downs138].
-
(2) Choice of diagnostic cut-off. The interpretation of the SICCT test relies on comparing the difference in size between the reaction to avian tuberculin and bovine tuberculin. Altering the cut off for a reactor from >4 mm size difference (standard interpretation) to >2 mm (severe interpretation) increases the sensitivity of the test and decreases specificity [Reference Ngandolo139].
-
(3) Rigorous test procedure. Qualitative research has identified several key issues that may influence the quality of testing, including on-farm conditions, tester training and experience, and the conflict of interest in the relationship between private veterinarians and the farmer [Reference Meskell, Devitt and More140]. Some breeds of cattle may pose testing difficulties that can cause decreased sensitivity, for example bullfighting cattle in Spain [Reference Alvarez85]. Considerable variation has been found between veterinarians in their application of the comparative test [Reference Humblet141], although this study was in Belgium, which has had ‘officially tuberculosis free’ status since 2003 and so TB testing would be relatively infrequent. Differences in test protocol have also been shown to influence the IFN- γ test [Reference Gormley142]. In Ethiopia, higher throughputs in abattoirs have been associated with lower lesion detection in routinely slaughtered cattle [Reference Shitaye, Tsegaye and Pavlik143].
-
(4) Disease stage in the animal. bTB is a chronic, slowly progressing disease. The sensitivity of abattoir surveillance based on detection of visible lesions increases with disease advancement [Reference Shitaye, Tsegaye and Pavlik143]. While the TST detects early immunological changes associated with infection, there is still a delay, with earliest detection being estimated at between 21 and 50 days post-infection [Reference Thom10, Reference Monaghan136]. During the late stages of the infection, particularly in severe and generalised disease, responsiveness to the skin test is reduced (anergy) [Reference Thom10, Reference Shitaye, Tsegaye and Pavlik143, Reference Monaghan144]. During anergy, the infection is likely to be systemic and severe, cattle are potentially more infectious thus increasing the significance of inadequate detection.
-
(5) Desensitization to the test. The majority of studies reviewed, but not all [Reference Thom10], have shown that the responsiveness to tuberculin is reduced for up to 60 days after the skin test [Reference Monaghan136, Reference Kerr, Lamont and McGirr145]. Some evidence suggests that a TST may increase the sensitivity of subsequent IFN- γ tests [Reference Ryan, Buddle and De Lisle146], although findings tend to vary [Reference Gormley142, Reference Rangen147]. Immunosuppression due to stress, comorbidity, advanced tuberculosis or calving may also lead to variation in reactivity to tests [Reference Pollock and Neill51, Reference Kerr, Lamont and McGirr145, Reference Huitema148, Reference Buddle149].
-
(6) Host or pathogen genetic variation. There is some evidence that some cattle may be genetically predisposed to pass the standard TST used in GB [Reference Amos150]. However, in an extensive study (21 000 isolates) in Northern Ireland, no association between skin test response and M. bovis genotype was detected, after controlling for confounding factors [Reference Wright151].
-
(7) Cross-reactivity. A diverse range of saprophytic mycobacteria exist in the environment, which although they rarely cause disease in cattle, are significant in immunological diagnosis. For example, M. avium [Reference Amadori152–Reference Barry154], M. fortuitum [Reference Michel155] and M. kansasii [Reference Waters156–Reference Vordermeier158], may all interfere with the interpretation of diagnostic tests for M. bovis. Such bacteria can cause animals to react to bovine tuberculin and hence be interpreted as a false-positive bTB diagnosis, or otherwise interfere with test interpretation [Reference Monaghan144, Reference Michel155, Reference Waters156, Reference Corner and Pearson159]. Conversely, it has been shown experimentally that a genuine positive response to bovine PPD can be masked by a larger or commensurate response to avian PPD [Reference Hope153, Reference Waters157, Reference Thom160, Reference Howard161], suggesting that M. bovis infection may be concealed for some time in cattle sensitized by mycobacteria of the avium/intracellulare complex, thus preventing detection and removal.
-
(8) Concurrent infection. There is accumulating evidence that concurrent infection with F. hepatica and with MAP may affect the performance of immunological tests for bTB infection. Evidence suggests the immunomodulatory effects of the helminth F. hepatica may reduce the sensitivity of immunological diagnostic tests for tuberculosis [Reference Flynn63, Reference Ezenwa65, Reference Elias109, Reference Flynn162]. Stronger skin responses to tuberculin tests have been reported in cattle that had been dewormed compared to animals that had not, although bTB infection status was not determined [Reference Ameni and Medhin163]. Cross-reactivity of some proteins shared between M. bovis and MAP, can impede diagnosis of bTB in concurrent infections [Reference McDonald164–Reference Seva168]. False-positive bTB diagnoses, by the caudal fold test, have been recorded in animals that tested positive for MAP either by the detection of MAP in their faeces or a positive ELISA [Reference Brito166]. Conversely, infection with MAP has been known to obscure the detection of M. bovis in concurrent infections in Spanish cattle [Reference Aranaz167]. Vaccination against MAP can interfere with immunological methods of bTB diagnosis in cattle [Reference Muskens169–Reference Coad172] although, in one study, animals vaccinated with an inactivated MAP vaccine in field trials showed cross-reactivity to the single intradermal tuberculin test (SIT) but not to the SICCT [Reference Garrido173]. Despite its limitations, serology has been used to distinguish TB-free cattle that have been vaccinated against Johne's disease [Reference Tewari174].
LANDSCAPE, CLIMATE AND OTHER ENVIRONMENTAL RISK FACTORS
Survival and persistence of M. bovis in the environment
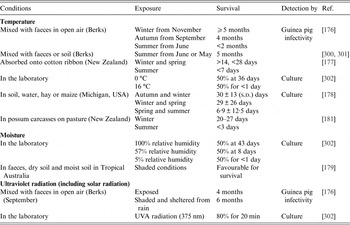
Estimates of environmental survival of M. bovis, evaluated for artificial and natural contamination of several sites and substrates are extremely variable, ranging from a few days to 2 years [Reference Courtenay and Wellington175] (Table 3). Much of the data derives from early studies in GB and other countries where levels of natural environmental contamination may have been high, given the abundance of clinically advanced bTB in cattle. In general, tubercle bacilli survive best in cool, moist environments shaded from direct sunlight and survival depends on temperature, sunlight and relative humidity (Table 3). Longer survival times in winter compared to summer months have been recorded for M. bovis in experimental studies conducted in GB [Reference Williams and Hoy176], New Zealand [Reference Jackson, deLisle and Morris177], Michigan [Reference Fine178] and Australia [Reference Duffield and Young179] (Table 3). M. bovis remained viable up to twice as long in shady conditions compared to sunny conditions [Reference Vera and Volkovsky180].
Table 3. The effect of environmental variables on the survival of M. bovis
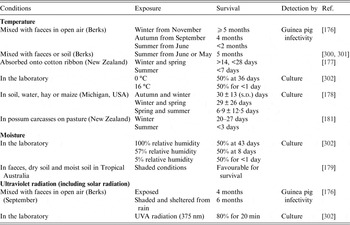
Experimental studies demonstrate that M. bovis can be isolated >5 months after inoculation when mixed with faeces [Reference Williams and Hoy176] and after one month in spiked possum carcasses [Reference Barron181]. Viable bacteria were recovered from hay at 7 days after inoculation and could still be isolated from samples of apples, corn and potatoes after 112 days [Reference Palmer and Whipple182]. In silage, the survival of M. bovis is likely to be shortened by the acidity (pH reduction) and temperature during fermentation. When silage containing large quantities of naturally infected faeces was fed to guinea pigs, it was infective for up to 10 weeks post-inoculation (Ulli Reuss, 1955 quoted in [Reference Goodchild and Clifton-Hadley80]).
Farm practices relating to both the storage and spreading of slurry can increase the risk of a herd bTB incident [Reference Ramirez-Villaescusa6, Reference Reilly and Courtenay93, Reference Griffin95, Reference Hahesy183] and the role of slurry in the transmission of bTB has recently been evaluated [Reference McCallan, McNair and Skuce184]. M. bovis can persist in slurry for up to 6 months [Reference Hahesy183, Reference Scanlon and Quinn185] and spreading of slurry after storage for <2 months has been associated with an increased risk of bTB [Reference Griffin105]. Experimental studies have demonstrated that M. bovis can remain viable for up to 12 h after aerosolization and the bacteria is resistant to stresses of being airborne [Reference Gannon, Hayes and Roe186]. Methods that reduce the distance over which slurry is dispersed and injecting slurry into soil, rather than spraying under pressure, reduces the risk of drift [Reference Hahesy183].
M. bovis has been recovered from soil sampled from badger setts and fields on a farm in Ireland that had a recent TB incident [Reference Young, Gormley and Wellington187] and was isolated from one water sample from yards frequented by infected badgers in England [Reference Little188]. In the United States, despite extensive sampling, M. bovis was not detected in soil, water, livestock bedding, feed, faeces, hay, pasture and grass samples collected from farms in Michigan and Texas recently positive for bTB [Reference Witmer189–Reference Pillai191]. However, prevalence in cattle and in local wildlife, including white-tailed deer (Odocoileus virginianus) (<2%), was much lower than apparent M. bovis prevalences in badgers that have been recorded in GB and Ireland (14–16%) and infection in cattle populations was also lower [Reference O'Brien192–Reference Murphy195].
Estimating the extent of environmental contamination with M. bovis may be confounded by the practical difficulties inherent to its isolation and culture from environmental samples [Reference Courtenay and Wellington175, Reference Fine190]. Procedures for concentrating bacteria from larger samples are required to compensate for the patchy distribution of bacteria in environmental samples [Reference Witmer189]. Molecular methods specific to M. bovis have been developed to detect and quantify mycobacterial DNA in environmental samples [Reference Sweeney196, Reference Parsons197] although they have limited sensitivity and positive results do not necessarily indicate viability or infectivity. On the other hand, DNA in dead cells did not survive beyond 10 days, suggesting that the DNA extracted from environmental samples may have come from intact cells [Reference Young, Gormley and Wellington187].
Weather and climate
Wint et al. [Reference Wint198] were able to predict the geographical distribution of bTB in England and Wales with a high level of precision (kappa = 0·68) using monthly remote-sensing data for weather-related variables (resolution 1–5 km) and cattle density. These variables were more important predictors than land use or vegetation. Areas with the greatest risk of bTB were more moist, having a lower water vapour pressure deficit (VPD). They also had their peak VPD later in the year and had lower variability of VPD and temperature. Recently, Jin et al. [Reference Jin299] were able to predict bTB incidence from rainfall over a 5-year period in an area of Wicklow, Ireland; the strongest correlations being with rainfall one or two quarters before the breakdowns. However, a previous study from Ireland failed to consistently predict the incidence of bTB reactors over 15 years using a range of weather variables [Reference Jin299]. The North Atlantic Oscillation (NAO), a major determinant of weather conditions in Western Europe, has been shown to be a significant correlate of culled badger M. bovis prevalence, but its inclusion in statistical models left a great deal of unexplained inter-annual variation which was better explained by the effect of the 2001 foot-and-mouth disease outbreak [Reference Woodroffe200].
Flood and drought could affect the risk of infection with bTB by influencing the survival of M. bovis (Table 3) or by affecting contact patterns between cattle and other cattle or infected wildlife but there is little published evidence for any relationship between flooding in temperate climates. It has been hypothesized that, in times of drought, badgers may resort to woodlands for prey in moist leaf litter and their contact with cattle would be reduced [Reference Hancox201]. In Africa, associations between increased bTB prevalence and flooding have been attributed to enforced contact between herds [Reference Cleaveland202], and with drought as it forces cattle to use communal water sources shared by infected and naïve cattle [Reference Balako203] and encourages large-scale movements [Reference Tadeusz and Bouazza204].
Landscape
A number of landscape and environmental factors have been found to be associated with increased or decreased herd bTB incidents, although the degree of association varies [Reference Reilly and Courtenay93, Reference White and Benhin100, Reference Winkler and Mathews116, Reference Mathews205–Reference Kaneene207].
Many landscape factors identified determine the suitability (or otherwise) of an area for badgers or act as barriers for the geographical spread of bTB [Reference Kelly and More208]. Landscape characteristics such as altitude and habitat composition are correlated with badger abundance [Reference White, Brown and Harris209, Reference Newton-Cross, White and Harris210]. Soil type has also been associated with badger abundance [Reference Neal211, Reference Hammond, McGrath and Martin212] with badgers preferring sandy loamy soils for sett construction. Interestingly, soil characteristics also correlate with bTB risk, with bTB-affected farms being less likely to have deep clay soils or seasonally wet soil [Reference Johnston70] and M. bovis was more likely to be isolated from sandy soil in cattle farms in Michigan [Reference Walter213]. This soil type may provide a moist, well drained microclimate that could maintain suitable pH and moisture levels that favour bacterial survival.
The distribution of badger latrines in a high density population in South-West England varied according to habitat types and landscape features [Reference Delahay214]. Latrines are generally more commonly found in woodland and are less common in arable or grassland [Reference Delahay214, Reference Hutchings, Service and Harris215]. A high proportion of latrines are located within 5 m of a linear feature (especially hedges and stone walls) [Reference Delahay214] or are associated with runs crossing such linear features [Reference White, Brown and Harris209].
Higher densities of hedgerows [Reference Mathews205] and a higher percentage of boundaries composed of hedgerows [Reference Winkler and Mathews116] on a farm were associated with a reduced risk of a bTB incident in the resident herd. The authors suggested that this was because longer forage (preferred by grazing cattle) is associated with higher hedgerow density and thicker hedgerows may mean that cattle cannot access areas that may be more contaminated with badger faeces and urine.
Water sources
Aggregation at communal water sources encourages closer contact between cattle and may increase the likelihood of contact with wildlife, although there is little evidence for a risk pathway in GB. Evidence from Spain found farms with high density of streams had a lower risk of TB, thought to arise because dispersed water resources reduced aggregation of cattle and wildlife [Reference Cowie115]. The presence of water sources (ponds and creeks) with uncontrolled access has been associated with an increased risk of bTB in Michigan, USA [Reference Kaneene207] and in Western Uganda [Reference Kazoora216].
Transmission through birds, invertebrates and protozoa
Mechanical transfer of M. bovis bacteria on the feet of birds [Reference Witmer189] or via invertebrates [Reference Fischer217] is considered a theoretical risk although has yet to be demonstrated. M. bovis has been isolated from various species of birds in Europe, although they were predominantly birds of prey [Reference Wilson218]. A report from the Soviet Union describes M. bovis and M. avium in several species of tick [Reference Blagodarnyi219]. M. bovis has also been detected in horn flies (Haematobia irritans) [Reference Torres220].
Experimental studies have demonstrated that M. bovis can survive in protozoa (Acanthamoeba castellanii), potentially facilitating transmission by extending the survival of the bacteria in the soil [Reference Taylor221]. However, co-incubation of both organisms under laboratory conditions substantially reduced levels of bacteria, suggesting that while there is the theoretical potential for environmental amoebae to act as a reservoir of M. bovis, they may also reduce environmental contamination [Reference Mardare, Delahay and Dale222]. Acid-fast microorganisms have been observed inside amoebae isolated from infected badger setts, although attempts to confirm them as M. bovis by culture or PCR were unsuccessful [Reference Mardare, Delahay and Dale222].
WILDLIFE
Badgers
In the UK and Ireland the European badger (Meles meles) is the main wildlife reservoir for bTB infection in cattle. A reservoir is defined as a epidemiologically connected population in which infection is permanently maintained and transmitted to a target population [Reference Haydon223]. Furthermore, this reservoir of infection in badgers is often cited as the main barrier to eradicating infection in domestic livestock in these countries. The most compelling evidence of the infection risk that badgers pose to cattle comes from large-scale trials in the UK and Ireland, where badger culling had significant impacts on the incidence of bTB in cattle [Reference Donnelly224–Reference Bourne227]. Within the boundaries of proactively culled areas during the Randomized Badger Culling Trial (RBCT) cattle bTB incidence was reduced by 33% after four annual rounds of proactive culling [Reference Bourne227]. Given that not all badgers were culled [Reference Smith and Cheeseman228], this is likely to represent a minimum number of incidents that are attributable to badgers in these areas. More recent analyses of the RBCT data suggests that in an endemic area, badgers are directly responsible for 6% of bTB infections in cattle and the overall contribution from badgers, through onward cattle to cattle transmission, is ~50% [Reference Donnelly and Nouvellet229]. How badgers transmit bTB to cattle is not known, although several likely routes are described below.
Proximity and abundance of badgers
Presence and abundance of badgers near cattle farms have been included in a range of analyses of potential bTB risk factors. Analyses of risk factors associated with cattle herd incidents that include the distance from farm buildings to badger setts have produced conflicting results. Johnston et al. [Reference Johnston71] found that including presence of occupied badger setts within 1 km of farm boundaries did not improve their bTB risk prediction models, and Griffin et al. [Reference Griffin88] likewise observed that the distance to badger setts did not vary relative to bTB incidents in herds. Conversely, Martin et al. [Reference Martin230] found a significant, though weak, reduction in the risk of an incident with increasing distance to badger setts and [Reference Denny and Wilesmith89] found positive associations between the presence of badgers and herd incidents in Northern Ireland. In South-West England, finding dead badgers on a farm was associated with a threefold increase in the risk of a bTB incident [Reference Johnston70].
Studies investigating cattle bTB risks in relation to local badger density have also produced mixed results. For example, Reilly & Courtenay [Reference Reilly and Courtenay93] reported an association between high densities of badger setts and persistent cattle herd incidents, but only after adjusting for farm management-related variables. Similarly, another study reported a correlation between the number of active badger setts within 1500 m of a farm and the probability of cattle herd incidents [Reference Vial, Johnston and Donnelly91]. However, a study by Mathews et al. [Reference Mathews205] found little evidence of an association between badger density and bTB risk, as farms with management practices that favoured wildlife had a lower risk of bTB.
M. bovis transmission routes
Badger-to-cattle transmission has been experimentally demonstrated [Reference Little188], but the principal routes of transmission in the field can only be inferred at present. Infected badgers may excrete M. bovis bacilli in urine, faeces, sputum and exudate from open abscesses [Reference Clifton-Hadley, Wilesmith and Stuart231]. Studies have identified M. bovis in soil and latrines [Reference Courtenay232], and there is evidence for bacilli remaining infectious for some time after being deposited, at least in certain environmental conditions [Reference Wilesmith233].
Potential routes of transmission include direct transfer via very close contact between badgers and cattle, and indirect contact where cattle encounter infectious material from badgers. Both mechanisms could theoretically occur at pasture or in farm buildings, but the relative importance of these transmission routes is unknown. As smaller numbers of bacilli are needed to infect cattle via the respiratory system than via the digestive system [Reference Humblet, Boschiroli and Saegerman50], inhalation of bacteria is likely to be the main route of infection [Reference Phillips234]. This could occur during nose-to-nose contact with badgers, but also while grazing, as cattle aerosolise and inhale bacilli on contaminated pasture or forage.
Direct transmission risks
Previous observational studies suggested that close contact between badgers and cattle at pasture is unlikely [Reference Benham and Broom235] and other research suggests that badgers may actively avoid pasture with grazing cattle [Reference Mullen236]. Studies using proximity loggers have shown that close contact between badgers and cattle does occur at pasture although it was reported as relatively infrequent [Reference Bohm, Hutchings and White61] or very rare [Reference O'Mahony81, Reference Drewe237]. The available evidence suggests that direct contact at pasture is unusual, although contact rates could vary across different landscapes. Badgers may frequently visit farm buildings, where they may readily come into close contact with cattle [Reference Garnett, Delahay and Roper119, Reference Judge238–Reference Tolhurst240]. The evidence from several studies suggests that direct contact may be more frequent in buildings and it has been suggested that it may be easier to reduce risks by modifying buildings to exclude badgers [Reference Judge238, Reference Ward, Judge and Delahay241].
Indirect transmission risks
Badgers habitually defecate and urinate at latrines [Reference Roper242] This may concentrate potentially infectious material and give rise to enhanced infection risk to cattle at certain locations, although it may conversely reduce risks elsewhere [Reference Smith243]. Early research suggested that cattle avoided grazing on pasture contaminated by badger faeces [Reference Benham and Broom235, Reference Benham244]. However, subsequent studies showed that cattle would explore contaminated pasture, and a small proportion would graze at latrines [Reference Hutchings and Harris245]. Visits by cattle to badger latrines recorded by proximity loggers found that 85% of the cattle in one herd actively investigated badger latrines and 15% of them visited latrines over 100 times in a 6-month period [Reference Drewe237]. The extent to which this occurs may be affected by farm management practices such as grazing rotation patterns [Reference Benham244] which in turn influence factors such as sward height [Reference Hutchings and Harris245].
Badger urine may present a significant bTB transmission risk to cattle as it can contain up to 300 000 bacilli/ml [Reference White, Brown and Harris209, Reference Gallagher and Horwill246]. Grazing cattle do not appear to avoid badger urine deposited away from latrines [Reference White, Brown and Harris209, Reference Brown, Cheeseman and Harris247], which often occurs on runs crossing linear features such as hedgerows [Reference White, Brown and Harris209]. Cattle have been shown to graze readily at such crossing points, and elsewhere on pasture contaminated with badger urine [Reference White, Brown and Harris209, Reference Hutchings and Harris245].
Badgers have been observed both defecating and urinating onto stored cattle feed in farm yards, buildings and cattle troughs [Reference Garnett, Delahay and Roper119, Reference Tolhurst240, Reference Garnett, Roper and Delahay248]. Cattle have been recorded showing little or no avoidance of feed contaminated with faeces from rodents and wild birds [Reference Daniels, Hutchings and Greig249]. It has been suggested that this non-selective feeding behaviour may represent a bTB transmission risk especially if the contaminated feed is well-mixed. One questionnaire-based study provided some support for this hypothesis, finding a significant positive association between the perceived reported presence of badgers in feed stores or cattle housing and an increased risk of cattle herd incidents [Reference Bourne227].
Wild boar
M. bovis infection has been isolated from wild boar in several European countries [Reference Pavlik250–Reference Santos257], including in a feral wild boar (Sus scrofa) in the UK [Reference Foyle, Delahay and Massei258]. Research on the Iberian Peninsula has reported bTB prevalence of up to 52·4% [Reference Gortazar259] and positive associations between the density of wild boar populations and cattle bTB incidence [Reference Gortazar260, Reference Vicente261]. The presence of wild boar on cattle farms in Spain, as reported in farmer questionnaires, was associated with TB breakdowns [Reference Cowie115]. Furthermore, the M. tuberculosis complex genotypes found in wild boar are of the same origin as the bovine and caprine genotypes found in domestic swine, deer and humans [Reference Gortazar259, Reference Aranaz262, Reference Parra263]. Hence, there is evidence to support the role of the wild boar as a reservoir host of M. bovis in Mediterranean areas (reviewed in [Reference Naranjo264]). In the UK, wild boar have a limited geographical distribution, mainly confined to small areas in Kent/Sussex and Gloucestershire/Herefordshire. Therefore, the current risk to livestock from this species in the UK is considered to be low, although this has the potential to change should wild boar numbers and geographical distribution change. There is currently no empirical data on the growth trajectory of wild boar populations in the UK. This species has a very high reproductive rate for an ungulate and hence the potential to increase population size rapidly [Reference Gaillard, Brandt and Jullien265], although hunting and restricted woodland cover may constrain their numbers and distribution in the UK [Reference Wilson266].
Deer
Several wild deer species in Europe have been found to be susceptible to bovine bTB infection, including fallow (Dama dama), roe (Caprolus capreolus), red (Cervus elaphus), sika (Cervus nippon), Reeves’ muntjac (Muntiacus reevesi), reindeer (Rangifer tarandus) and elk (Alces alces) [Reference Wilson218]. Spatial associations between strain types in deer and cattle have been demonstrated by restriction fragment-length polymorphisms in Ireland [Reference Skuce267] and by spoligotyping in Spain [Reference Aranaz268], suggesting that bTB is transmitted between these species. Furthermore, experimental studies have demonstrated that indirect transmission of bTB could occur between white-tailed deer (Odocoileus virginianus) and cattle through sharing of feed [Reference Palmer, Waters and Whipple269]. Spatially explicit modelling of density and distribution of white-tailed deer and cattle herds with bTB breakdowns in Michigan, USA, demonstrated that infected deer play an important role in the maintenance of bTB in that area [Reference Walter213].
In a case control study in Michigan, USA, Kaneene et al. [Reference Kaneene207] found that the prevalence of bTB in the local deer population was an important risk factor for bTB in cattle. They also found that bTB risk was increased when water was provided to cattle outdoors and large quantities of hay bales were stored in fields or on pasture fence lines. Conversely, the risk was reduced if feed was stored indoors or properly protected by bagging or wrapping. A semi-quantitative assessment of the risk of transmission from deer to cattle in the UK, based on prevalence of infection, extent of potential bacterial excretion, likelihood of contact with cattle and approximate biomass suggested that red and particularly fallow deer represented the greatest potential risk. However, any risk is likely to be localised given the geographical variation in deer densities [Reference Delahay270].
Other species
M. bovis has a wide host range and is the most common cause of tuberculosis in mammal species [Reference Delahay271]. M. bovis infection has been identified in numerous wild mammal species in the UK (see [Reference Delahay271]), although a semi-quantitative risk assessment suggested that species other than deer and badgers are unlikely to pose a significant risk of bTB transmission to cattle [Reference Delahay270].
HUMAN-TO-CATTLE TRANSMISSION
There are a large number of documented cases of human to cattle transmission from the first part of the 20th century in Europe, in Denmark [Reference Nielsen and Plum272], the UK [Reference Blacklock273], Germany [Reference Wiesmann274–Reference Wolter, Schulz and Siering277], The Netherlands [Reference Huitema148]. However a case was reported relatively recently in Switzerland [Reference Fritsche278]. More recently, associations between tuberculosis in humans and cattle have been predominantly documented in Africa [Reference Cleaveland202, Reference Fetene, Kebede and Alem279–Reference Awah-Ndukum282].
DISCUSSION
We present an overview of risk factors that have been identified for bTB in cattle, with a primary focus on the UK and Ireland, but where gaps exist drawing on evidence from further afield. However, there are difficulties in extrapolating from many studies and in weighting the relative importance of the various risk factors identified in the literature owing to differences in study design, circumstances, follow-up time, selection criteria and case definitions. Variation in herd composition, management, history of infection and local circumstances, including exposure to potential sources of infection from wildlife, will all vary in space and time creating serious challenges for between study comparisons. The case definition for a bTB-infected herd in Europe is detection of one or more reactors to the SIT or SICCT test (64/432/EEC) at standard interpretation but definitions in the literature range from other interpretations of the skin test [Reference Gumi44, Reference Moiane48, Reference Reilly and Courtenay93] to laboratory-confirmed M. bovis infection [Reference Kaneene207], or to the detection of reactors with evidence of infection [Reference Denny and Wilesmith89]. The principal outcomes examined also varied from detection of infection to disease characteristics such as duration [Reference Karolemeas123], chronic persistence [Reference Guta92] and recurrence [Reference Doyle82, Reference Gallagher83, Reference Karolemeas124] (Table 1). This range supports the use of a review of evidence from several studies looking at similar research questions over a relatively broad geographical range.
Nevertheless, despite differences in study design some clear and consistent patterns have emerged across a broad range of infection prevalence and environments. Prominent are the increased risks associated with animal age, contact with a wildlife reservoir and the size of the herd. Purchasing strategies and management practices that favour intensive production, also promote increased contact among cattle (housing) and with wildlife (fragmentation, farm size) at the expense of hygiene and biosecurity. Herds with a history of bTB are consistently identified as being at higher risk of a future bTB incident. The relative importance of different risk factors also varies according to incidence. In low-incidence areas, infection is predominantly related to cattle movements [Reference Humblet103, Reference Gates, Volkova and Woolhouse106]. In higher risk areas, risk factors reflect increasing contact rates, as is unsurprising for a contagious disease, e.g. herd size, farm size, movements, housing, and exposure to wildlife (Table 1).
Causality between markers for infection or disease and many risk factors cannot be assumed. Many risk factors may act as proxies for contact and transmission opportunities or other unmeasured variables. Landscape characteristics will influence the distribution and abundance of wildlife and reflect different cattle management practices, and so may be proxies for contact rates among cattle and between cattle and wildlife. Also, the quality of animal husbandry may affect the level of M. bovis contamination and attitudes to biosecurity. Hence, the significance of the reported protective association between vitamin and mineral supplementation and bTB [Reference Ramirez-Villaescusa6, Reference Downs55, Reference Griffin95, Reference Christiansen and Clifton-Hadley114] may be a proxy for poor management and hygiene practices on infected farms. More research is needed to determine the impact and relative contribution of management factors, such as nutrition and housing on infection transmission and pathogenesis within the host animal.
The risk that bacilli released into the environment by infected cattle or wildlife will infect other animals depends on the rate at which they lose their viability and virulence. Survival of M. bovis in soil and other environmental substrates depends on temperature, moisture, pH, exposure to sunlight, oxygenation, and interactions with other microflora, and so will be expected to vary widely in space and time. Such environmental factors have been associated with variation in the risk of bTB in cattle [Reference Hahesy183].
Knowledge gaps and the way forward
The relative importance of the risk factors identified to date are likely to vary among farms and environments, and over time making it difficult to identify generic patterns. Many risk factors have been identified in relation to different types of herd management such as sharing of equipment [Reference Cowie115] and types of housing [Reference Johnston71, Reference Griffin95], but their impact is unknown. In many studies once location, bTB history and herd size are accounted for, the degree to which management factors may influence the risk of an incident is low. The calculation of the population attributable fraction in bTB risk factor studies is relatively rare, but can provide an assessment of the absolute contribution of a risk factor to infection incidence or prevalence [Reference Johnston71, Reference White96, Reference Downs138]. It can be used to provide a measure of how many bTB cases might be prevented by modifying or removing a risk factor or range of factors. If prevalence of the exposure is low, then the relative importance of the risk factor will be low, even if the association with disease is quite strong.
Few risk factor analyses have matched cases and controls on herd size [Reference Johnston70] but the dominance of herd size in many risk factor studies (Table 1) [Reference Skuce, Allen and McDowell73, Reference Vial75, Reference Brooks-Pollock and Keeling283], supports a case for conducting studies matched on herd size, to attempt to tease out other management characteristics that may be a feature of large herds and may promote infection transmission.
In the UK and Ireland there is a large body of evidence to support transmission from badgers to cattle [Reference Donnelly224–Reference Bourne227, Reference Martin230, Reference Group284] and transmission is likely to be reciprocal [Reference Woodroffe285] suggesting that in at least some circumstances the two populations may constitute a host community in which M. bovis circulates. Hence there is the potential for interactions and inter-dependency among cattle management and wildlife-related risk factors. The potential impact of transmission from badgers to cattle has been estimated from the RBCT at ~33% [Reference Bourne227] although identification of the most important routes of transmission remains unresolved, thus hampering the development of targeted control measures.
In GB, mathematical modelling supports other work showing cattle movements, re-infection from wildlife and the performance of the main diagnostic test have a substantial influence on infection incidence [Reference Brooks-Pollock, Roberts and Keeling286]. Currently approved diagnostic tests for bTB are known to be variable in their performance and have only moderate sensitivity [135]. Significant improvements in diagnostic test accuracy and hence case-definition could improve the estimation of the impact of risk factors, transmission rates and hence the development of appropriate control strategies. It has been estimated through modelling that up to 24% of British herds had residual infection in at least one animal when restrictions were lifted [Reference Conlan76]. This is congruent with estimates from a descriptive analysis in Spain, where 22% of incidents were determined to have been caused by residual cattle infection [Reference Guta287]. There is evidence that the TST is influenced in the presence of Johne's disease [Reference Alvarez29] and liver fluke [Reference Flynn63, Reference Flynn162] but the extent to which this might impede diagnosis is unknown. Potential impacts could be significant as the herd-level seroprevalence of Johne's disease in South-West England is high [Reference Woodbine288]. The geographical distributions of liver fluke and bTB infections are negatively correlated in England and Wales and it has been suggested that this is a result of masking of infection [Reference Claridge289]. However the highest prevalence areas for liver fluke, such as Cumbria in Northern England [Reference McCann, Baylis and Williams290] are not associated with higher than average detection of bTB infection by meat inspection, and bTB incidence remains low [84].
Accumulating evidence suggests that the susceptibility of cattle to M. bovis infection can be affected by breed and genotype but further work is required. About 20% of variation in resistance to bTB in British and Irish dairy cattle can be attributed to sire lineage, and there is evidence of similar variation in resistance in beef breeds. However, these may be under-estimates of heritability because of misclassification of estimated exposure to infection and imperfect diagnostic tests [Reference Bishop and Woolliams291]. This approach of breeding resistance is being taken forward by the dairy industry in GB [292].
The risk from within farm movements of animals and herds, although hypothesised, has thus far not been investigated. It is rare for this data to be captured centrally and so investigation would require a bespoke study.
The identification of tractable risk factors for bTB is critical to a better understanding of infection dynamics and the development of cost-effective approaches to disease control. While a cattle farmer can alter their behaviour or farm management strategies including purchasing choices, it is less likely or more challenging to exert substantial control over extrinsic factors related to the environment or wildlife, or the level of infection in the surrounding area. In recognition of this there is a need to account for farmer behaviour and the drivers of their decisions in the development of infection control strategies [Reference Leach and Scoones293, Reference Catley, Alders and Wood294]. Few studies have related farmer perceptions to bTB incidence, and those that have shown conflicting results [Reference Cowie115, Reference Cowie117, Reference Broughan295].
Knowledge of the importance of risk factors could be deployed to facilitate integrated control in two ways. First, certain risk factors may be shared among a range of pathogens of cattle although disease control in livestock usually focusses on individual infections. The identification of common risk factors should be used to develop more efficient, cost-effective holistic infection control programs. The identification of shared risk factors for brucellosis and bTB has indicated that such an approach is possible [Reference Cowie117]. Second, the combination of country- and region-specific risk factors could be used to focus on multiple transmission pathways simultaneously to continuously improve a more effective, integrated and evidence-based control strategy. Such an approach would need to be carefully validated and its effects would need to be measured.
If R 0 is just >1, changes in factors with a moderate effect on transmission will have a big impact [Reference Cox296]. Modelling approaches suggest that focusing on a single route of transmission will not reverse the increasing trend in incidence [Reference Brooks-Pollock, Roberts and Keeling286]. This suggests a tailored package of control measures, addressing many transmission pathways, made possible by the identification of relevant risk factors is likely to be the most effective approach to bTB control in cattle.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S095026881600131X.
ACKNOWLEDGEMENTS
This review was funded by the UK Department for Environment, Food and Rural Affairs (Defra) under project SB4500. The authors thank Professor Dirk Pfeiffer (RVC), Drs Simon Rolfe (Welsh Government), Martyn Blissitt (Scottish Government) Ricardo de la Rua Domenech, Malla Hovi and James McCormack (Defra, UK), and Ms. Kate Harris (APHA, UK), who read and made comments on drafts at various stages. Thanks to Dr Gareth Enticott, Cardiff University, for comments regarding social risk factors, and to Liz Pritchard, Cameron Smith, Andrew Petit (APHA Library), and Mary O'Mara (APHA) for administrative support.
DECLARATION OF INTEREST
None.