Introduction
Viral hepatitis is a leading cause of death worldwide, and more than half of the viral hepatitis deaths in China are caused by hepatitis B virus (HBV) [Reference Stanaway1]. It is well known that chronic HBV infection results in continuous liver injury and sometimes life-threatening complications, especially cirrhosis and hepatocellular carcinoma (HCC) [Reference Mokdad2, 3]. With an estimated 257 million people infected with HBV globally, 2–10% of these patients will progress to cirrhosis, and 0.2–0.6% of them will eventually develop HCC [4, Reference Fattovich, Bortolotti and Donato5]. The wide availability of antiviral drugs has significantly decreased the incidence rate of HBV-related cirrhosis and HCC [Reference Chang6–Reference Triolo, Della Corte and Colombo9]. However, liver disease progression and HCC development in patients with chronic hepatitis B (CHB) cannot be eliminated completely even in patients taking long-term nucleos(t)ide analogue (NA) therapy [Reference Triolo, Della Corte and Colombo9–Reference Hosaka11]. So, monitoring of disease progression in CHB patients taking antiviral therapy is still critical.
The development of cirrhosis and HCC in patients with CHB is complicated, dependent on numerous related factors including the host, virus and their interactions [Reference Iloeje, Yang and Chen12, Reference Yang13]. Older age and male gender are both strongly associated with the development of cirrhosis and HCC [Reference Fattovich, Bortolotti and Donato5]. Some studies demonstrate that elevated HBV DNA level is an independent risk factor for cirrhosis and HCC in CHB patients receiving no NA therapy [Reference Iloeje14–Reference Chien16]. High level of hepatitis B surface antigen (HBsAg) at diagnosis is also a risk factor for progression to HCC [Reference Chien16].
In this retrospective hospital-based, long-term study, we followed up a total of 6688 individuals with NA-treated CHB patients who had clinical records in the hospital information system (HIS). After starting NA treatment, levels of alanine aminotransferase (ALT), HBV DNA, HBsAg, hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg) and hepatitis B e antibody (HBeAb) were consistently recorded in these patients. The goal of the study was to describe the ‘real-world’ antiviral NA treatment patterns, to evaluate treatment responses and to assess possible factors that were related to the development of cirrhosis and HCC in a CHB cohort receiving NA therapy.
Methods
Data source
The study was conducted in the Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, which is a tertiary general hospital with more than 2000 beds in a first-tier city in China. It provides integrated healthcare services and represents one of the highest standards of care for patients with CHB in China. All physicians in the department are specialists in infectious disease and provide professional care for this disease area. The hospital established the HIS more than 18 years ago. Patients' data from various departments are linked through the unique patient identifier and are all stored in the HIS. Major clinical information, including patient demographics (birth year/month, gender, residency), visit dates, outpatient diagnoses, inpatient admission and discharge diagnoses, inpatient and outpatient treatment, procedures, laboratory test results and other diagnostic tests have been de-identified and transferred from the HIS into the research database. Diagnoses in the HIS database are coded using the International Classification of Diseases 10th revision (ICD-10).
Study design
This was a retrospective cohort database study to describe patient characteristics and disease consequences among patients with CHB who received antiviral treatment in the Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, China. Included patients were followed up from the earliest antiviral treatment date until the last visit date in the database. All clinical records of these patients, including data available back to the year 2000, were identified and extracted from the HIS. Antiviral medications extracted from prescription claims included lamivudine, adefovir, telbivudine and entecavir as mono- or combined therapies. This study was approved by the local Human Ethics Committee.
Study population
Patients aged at least 18 years who had a diagnosis of CHB in the Department of Infectious Diseases from 1 January 2011 to 31 December 2016, who received continuous care (at least three consecutive visits in the outpatient clinic or hospitalisation with no gap longer than 90 days between visits) and NA antiviral treatment, were included in this study. Exclusion criteria were as follows: at least with one diagnosis record of hepatitis C, hepatitis D, hepatitis G or human immunodeficiency virus (HIV), or pre-existing liver cirrhosis or HCC in the database before the earliest antiviral treatment date. All patients had at least one HBV-related laboratory test during the study period.
Laboratory tests
Laboratory test results for ALT, HBsAg, HBsAb, HBeAg, HBeAb and HBV DNA were captured from the HIS during the follow-up period. Serum HBV DNA quantification was determined using Applied Biosystems Real-time PCR system (Prism 7500; Applied Biosystems, Inc., USA, with a low limit of 500 IU/ml) or Roche COBAS HBV Amplicor MonitorTM assay (Roche Diagnostics, Basel, Switzerland; with a low limit of 20 IU/mL). Serum HBsAg, HBsAb, HBeAg and HBeAb were determined using commercial enzyme immunoassay kits (AXSYM System; Abbott, Wiesbaden, Germany). HBsAg was quantified by Abbott Architect HBsAg Reagent Kit (Abbott Ireland Diagnostics Division, Sligo, Ireland; dynamic range 0.05–250.0 IU/ml).
Definition of events
NA treatment duration and treatment compliance of patients were determined. Treatment duration was the time from the treatment index date until a treatment gap occurred (more than 121 days without NA) or the end of the study, whichever is earlier. Treatment compliance is the total time on drug within 365 days post the treatment index date divided by the total time in the database in the first 365 days post the treatment index date.
Patient serological indices and diagnosis, including biological breakthrough (BBT), virological breakthrough (VBT), HBsAg loss or seroconversion, HBeAg loss or seroconversion, functional cure, as well as cirrhosis and HCC, were captured during the cohort follow-up. BBT was defined as detecting ALT >5 times upper limit of normal in patients whose ALT had been normalised, or ALT >5 times nadir in those whose ALT level was continuously abnormal. VBT was defined as any increase in HBV DNA by >1 log10 from nadir, or ⩾10-fold the lower limit of detection of the HBV DNA assay. Seroconversion of HBsAg or HBeAg was defined as the development of HBsAb or HBeAb positivity in addition to loss of antigen, respectively. Functional cure was defined as loss of HBsAg with maintained virological remission (HBV DNA <2000 IU/ml) and ALT normalisation.
Statistical analysis
The person-years of follow-up were calculated from the date of the earliest NA treatment until the date of the relevant event (cirrhosis or HCC), the last visit date or the end date of study (31 December 2016), whichever was earliest. Incidence rates were calculated as the number of new cases divided by person-years of follow-up. When analysing the effect of serological changes on the incidence of cirrhosis or HCC, occurrence of cirrhosis or HCC within 6 months of serological change was not counted, in order to exclude those pre-existing cases with delayed diagnosis. For the same reason, in the patients who did not have serological change, the occurrence of cirrhosis or HCC within 6 months from initial NA treatment was also excluded. Cox proportional hazard regression models were utilised in comparative analyses and hazard ratios (HR) and their 95% confidence intervals (CI) were estimated. Age and gender at baseline were considered as potential confounders and have been adjusted for in the Cox proportional hazard regression model. All analyses were conducted using SAS version 9.4 (Cary, NC, USA).
Results
There were 9848 patients with CHB infection identified from the HIS who received NA treatment, continuous care and met eligibility criteria (Fig. 1). There were 113 patients who were excluded since they have been diagnosed with HCC before the date of the first NA treatment, 1185 had cirrhosis and 56 patients had been diagnosed with co-infections with hepatitis C, hepatitis D, hepatitis G or HIV. Of the remaining 8494 patients, there were 6688 patients who had at least one HBV-related laboratory test available for the main analysis.
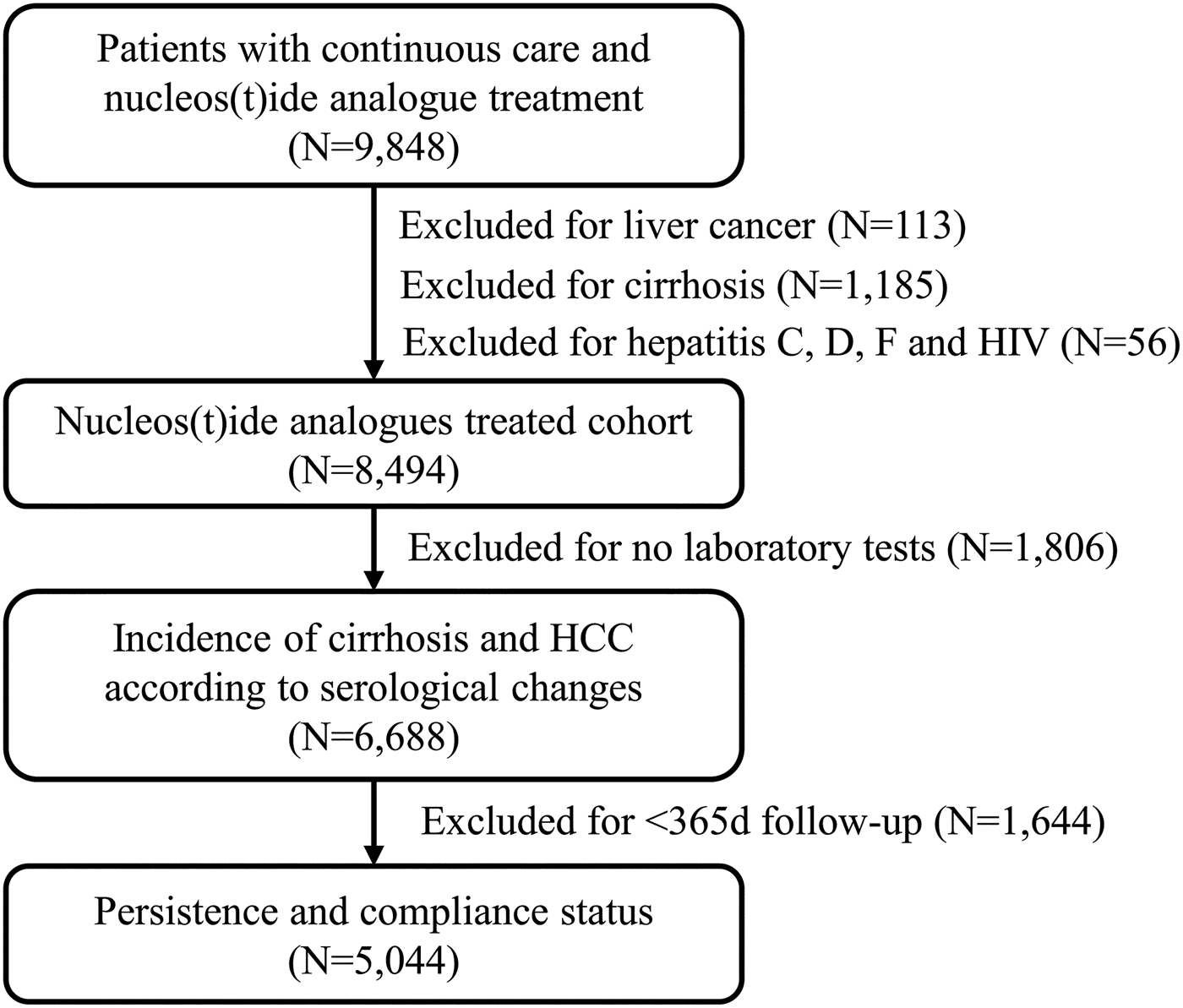
Fig. 1. Flow chart of the study cohort
Baseline demographic characteristics
The average follow-up time of the 6688 patients included in the study was 2.2 years (standard deviation (s.d.) 1.3 years). Characteristics of the CHB patients at our centre are shown in Table 1. More than half of the patients were male (n = 4422, 66.1%) and the age of the patients ranged from 18 to 97 years, with most between 30 and 39 years of age (n = 1912, 28.6%). A total of 3021 patients (45.2%) were local residents in Shanghai. Hypertension and diabetes were the most common comorbidities at cohort entry. The mean duration of NA therapy was 416.2 days (s.d. 437.0) (Table 2). The most common initial treatment was entecavir monotherapy (55.0%), followed by lamivudine monotherapy (16.0%) and telbivudine monotherapy (14.6%). Adefovir monotherapy (5.4%) was uncommon and only 9.1% of patients received combined therapy. Patients had a mean of 27.9 (s.d. 29.4) healthcare visits at the hospital (inpatient or outpatient for any reason) during the study (Table 2).
Table 1. Demographic and clinical characteristics at study entry in the chronic hepatitis B cohort study

s.d., standard deviation.
Table 2. Treatment characteristics in the chronic hepatitis B cohort, 2010–2016
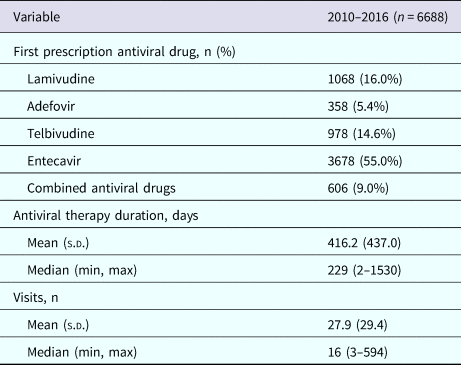
s.d., standard deviation.
Changes in the distribution of NA agents
With the admission of new drugs into the Chinese market, initial NA therapy for CHB patients under care in the hospital changed significantly over the study period. Lamivudine was the first antiviral drug available in the Chinese market, but gradually with the appearance of NA drugs which were more potent and had higher resistance barrier, lamivudine became a second-line drug in the clinical guidelines. As a result, lamivudine prescribing dropped 41% between 2011 and 2016 (64.7% in 2011 vs. 4.6% in 2016) annually. On the other hand, entecavir was the only first-line NA antiviral drug in the Chinese market until 2016. In our study, we observed a parallel increase in entecavir prescriptions from 22.4% in 2011 to 72.7% in 2016. Additionally, prescriptions of combined antiviral NA therapy decreased in CHB patients over these years (Fig. 2).
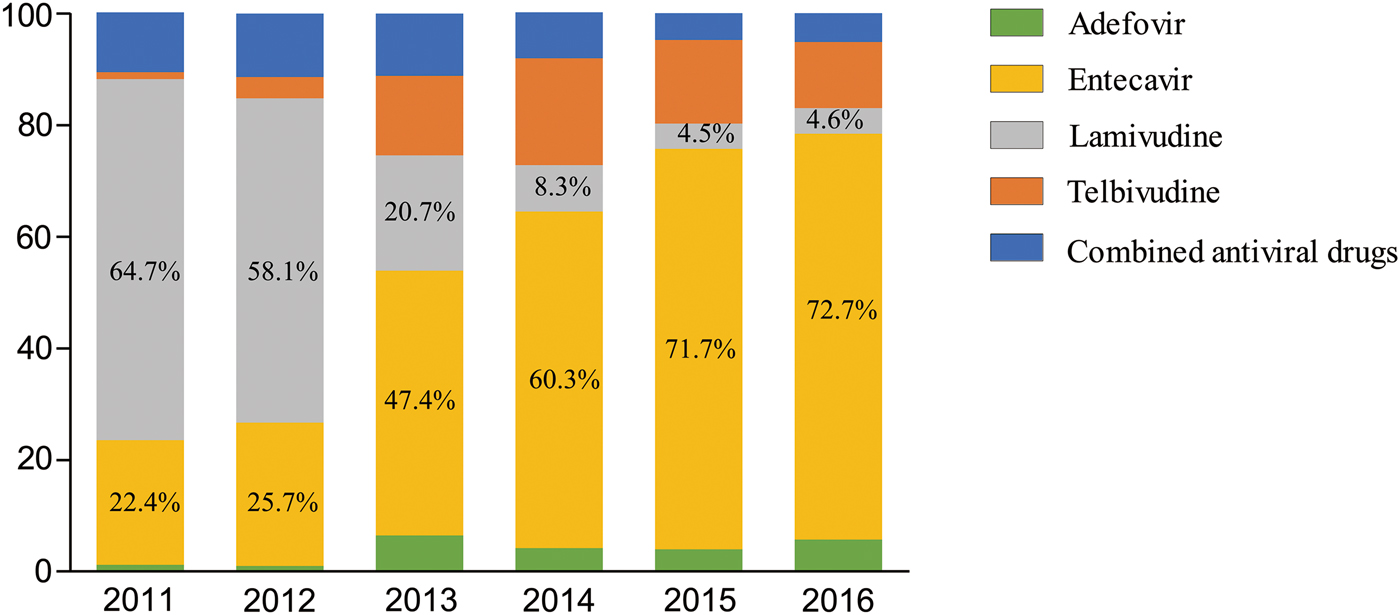
Fig. 2. Percentage drug distribution in patients with nucleos(t)ide analogues from year 2011 to year 2016
Associations between treatment patterns and progression to advanced liver diseases
In our study cohort, there were 1644 patients with <365 days of follow-up and these patients were excluded from the analysis of the effect of treatment duration and compliance on the incidence of cirrhosis or HCC (Fig. 1). Of the rest patients, 2582/5044 (51.2%) had good treatment duration (i.e. received NA treatment for at least 365 days), and 2800 (55.5%) were compliant with treatment (i.e. ⩾80% compliant with NA treatment in the first year of the follow-up period) (Table 3). The incidence of cirrhosis and HCC was significantly higher in patients with shorter treatment duration (adjusted HR 1.42, 95% CI 1.18–1.71 for cirrhosis and adjusted HR 1.96, 95% CI 1.23–3.13 for HCC). Similarly, the incidences of cirrhosis and HCC were also significantly higher in patients who were not compliant with treatment (adjusted HR 1.49, 95% CI 1.24–1.79 for cirrhosis and adjusted HR 2.12, 95% CI 1.33–3.38 for HCC).
Table 3. Incidence rates of cirrhosis and hepatocellular carcinoma in antiviral CHB cohort by treatment duration and compliance

a Adjusted for age and gender. P-value = χ 2 test.
HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma.
Associations between serological changes and progression to cirrhosis
There were 562 patients diagnosed with cirrhosis during follow-up in the NAs CHB cohort (incidence 41.0 per 1000 person-years). However, only patients with cirrhosis diagnosed at least 183 days after the serological change were considered as outcomes for the analysis in Table 4. None of the 53 patients with HBsAg loss and 26 patients with HBsAg seroconversion, or the 50 patients with a functional cure was diagnosed with cirrhosis during the study period. No significant associations were identified between BBT, VBT, HBeAg loss/seroconversion and the occurrence of cirrhosis.
Table 4. Number and incidence of patients who developed chronic hepatitis B related cirrhosis, diagnosed at least 183 days after the serological event

a Patients with cirrhosis diagnosed in the first 183 days (6 months) after the serological event were not counted.
b Adjusted for age and gender.
HR, hazard ratio; CI, confidence interval; BBT, biological breakthrough; VBT, virological breakthrough; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen.
Associations between serological changes and progression to HCC
There were 99 patients diagnosed with HCC during the study follow-up period (incidence of 6.8 per 1000 person-years). Possible risk factors that could affect HCC development were explored in patients diagnosed with HCC at least 183 days after the serological change (Table 5). In our study, cirrhosis was identified as the highest risk factor for HCC (adjusted HR 15.86, 95% CI 7.35–34.24). There were no cases of HCC among all 54 patients with HBsAg loss, none among all 27 patients with HBsAg seroconversion and none among all 51 patients who experienced a functional cure. However, there were no significant associations between BBT, VBT, HBeAg loss/seroconversion and the occurrence of HCC.
Table 5. Number and incidence of patients who developed hepatocellular carcinoma, diagnosed at least 183 days after the serological event

a Patients with HCC diagnosed in the first 183 days (6 months) after the serological events were not counted.
b Adjusted for age and gender.
HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; BBT, biological breakthrough; VBT, virological breakthrough; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen.
Discussion
This retrospective study comprehensively describes current antiviral treatment duration and compliance of CHB patients as well as possible serological factors which may correlate with the development of cirrhosis and HCC. The large sample size and longitudinal follow-up of the study cohort enabled the analysis of clinical outcomes that usually require years to develop, which gave us much more insights of what NA treatment can achieve in a real-world setting in those patients with CHB. In the current study, treatment duration and treatment compliance to antiviral NA drugs significantly affected the development of cirrhosis and HCC in patients with CHB. In addition, HBsAg loss may be a protective factor for developing cirrhosis or HCC. As expected, cirrhosis was still a significant risk factor for HCC development even in NA-treated patients.
Based on clinical practice and experience, entecavir and tenofovir were recommended as the first-line antiviral drugs for CHB in the guideline [17], which owned to their good efficacy and high resistance barrier. Changes of trend in NA treatment over years in our study were consistent with the changes in the guideline and reflect the changes in the management of patients with CHB in the Chinese population.
Current guidelines suggest continuous NA treatment, in patients with active CHB disease. Therapies that require continuous treatment are expected to result in poor compliance and some patients even giving up on treatment. Poor compliance is reported to be associated with an increased risk of virological failure [Reference Lieveld18]. In our real-world study, we showed that treatment duration and compliance had a significant effect on CHB outcomes during the follow-up period, with a lower risk of developing cirrhosis and HCC among patients who showed good compliance with therapy and longer treatment duration. It suggested that patients with antiviral therapy should be monitored regularly for a better outcome. However, patients with >365 days treatment duration in this study had a much longer follow-up period than the patients with <365 days treatment duration. Patients with longer treatment duration probably preferred receiving various healthcare services in the same hospital, which led to a longer follow-up period. When calculating the incidence rates, both person-years and number of cases may be overestimated simultaneously, which might cause potential minor bias.
Underlying liver diseases, including chronic viral hepatitis, can contribute to the development of liver cirrhosis [Reference Beste19]. Although not all patients with cirrhosis are certain to develop HCC, there are strong links between the two pathologies [Reference Fattovich20]. This has been described for patients with CHB in whom cirrhosis can predict the incidence of HCC [Reference Ieluzzi21], and in a study that showed that in the absence of antiviral therapy, the rate of HCC was 22-fold higher among CHB patients with cirrhosis compared to those without [Reference Chen22]. Sub-classification of cirrhosis even could be used as a significant predictor of late recurrence in patients with HBV-related HCC after surgery [Reference Kim23]. Our study confirms an important role of cirrhosis in HCC development; cirrhosis was associated with over a 15-fold evaluated risk to develop HCC in patients receiving antiviral therapy.
In patients with CHB, the rate of HBsAg loss ranges from 0.0% to 3.0% annually with entecavir or tenofovir treatment [Reference Terrault24], and an HBsAg loss rate of 8.4‰ annually was observed in our NA-treated CHB cohort. HCC can develop even after HBsAg loss in the absence of antiviral treatment [Reference Liu25]. However, no cases of HCC after HBsAg loss during antiviral therapy were observed in our real-world retrospective study, even no cirrhosis cases occurred. Our findings were similar with the Korean study, which shows a lower incidence of HCC in patients with HBsAg loss compared to patients without HBsAg loss for patients with CHB taking antiviral therapy over 6 years [Reference Kim26]. Our further study will be researched with a larger number of cases in the future, in order to provide strong evidence to illustrate the role of HBsAg loss or seroconversion in HCC or cirrhosis.
The predictive value of HBeAg as a marker of disease progression in patients with CHB is also controversial. The Kawerau township study in Maori patients found that HBeAg positivity was predictive neither for liver cirrhosis, nor HCC[Reference Lim27], while conversely, a study in Taiwan showed that HBeAg positivity could be considered as a risk factor for HCC [Reference Yang28]. In another study of antiviral-treated patients with CHB, no difference in the HCC incidence rate was reported between HBeAg-positive and -negative patients [Reference Cho29]. Consistent with these observations, our study showed that HBeAg loss or seroconversion did not prevent the development of cirrhosis or HCC. No association was found between HBeAg loss and HCC, which may be due to our relatively small HCC cases. Accordingly, the role of HBeAg loss or seroconversion in the development of cirrhosis and HCC in CHB patients with antiviral treatment remains ambiguous. Additional studies are needed to confirm these trends in antiviral-treatment patients.
Nowadays HBV DNA undetectable and ALT normalisation are the basic requirements with NA treatment. The REVEAL-HBV cohort study in Taiwan showed that increased HBV DNA and ALT levels were predictive for cirrhosis and HCC risk in patients not taking antiviral treatment [Reference Lee15,Reference Zoutendijk30,Reference Sinn31]. However, in our study, we found no associations between VBT and BBT with cirrhosis or the development of HCC. This discrepancy might reflect key differences between the study populations, whereby the patients in our study were treated with antiviral therapy, compared with no antiviral use in REVEAL-HBV. The predictive value of HBV DNA and ALT levels may be weakened with the application of antiviral therapy.
However, there are also limitations to our study. As this study was a retrospective study based on HIS in a tertiary hospital, aspects such as lifestyle and family history, including HCC family history, smoking, alcohol assumption and diet were not captured in the current study. In addition, there might also be immortal time bias in the study for those patients who did not have serological change because their follow-up period is overestimated in the study.
In conclusion, this study constructed a large antiviral-treated CHB cohort for the analysis of possible risk factors which could affect the development of cirrhosis and HCC. The use of real-world data provided a unique insight into the effect of treatment duration and compliance on clinical outcome. Patients with CHB should be encouraged to adhere to NA therapy in view of the positive impact of good compliance on cirrhosis and HCC risk. Meanwhile, potential protective effects of HBsAg loss on the development of cirrhosis and HCC warrant further investigation. In line with some earlier studies, cirrhosis was still identified as an independent risk factor for HCC in NA-treated patients.
Author ORCIDs
Y. Sun, 0000-0002-4784-9189; W. Cai, 0000-0001-9324-5987
Acknowledgements
This study was funded by the National Natural Science Foundation of China (81470867), the National Thirteenth Five Year Plan Major Special Project (2017ZX09304016), the National Science and Technology Major Project of China (2014ZX10002002), the Shanghai Municipal Key Clinical Specialty (Infectious diseases), the Shanghai Three-Year Plan for Clinical Skills and Clinical Innovations (16CR1002A).
Conflict of interest
Y. Zhang, M. Shu, K. Bonroy and H. Qiu are employees of Janssen Research & Development. Y. Zhang, K. Bonroy and H. Qiu report stock ownership in Johnson and Johnson. Other authors declare no conflicts of interest.









