INTRODUCTION
Achromobacter spp. are opportunistic pathogens increasingly recovered from adult patients with cystic fibrosis (CF) [Reference Ciofu, Hansen and Høiby1]. However, owing to difficulties of accurate species identification, most clinical Achromobacter isolates are often reported as A. xylosoxidans. The reported prevalence of airway colonization/infection by A. xylosoxidans in CF centres ranges from 2% to 20% [Reference Tan2, Reference De3] but their clinical significance remains unclear. Nevertheless, a retrospective case-control study [Reference Ronne-Hansen4] showed a greater decline in lung function in patients chronically infected with A. xylosoxidans, compared to non-infected patients. The clinical course of CF lung disease is widely considered to be dependent on the nature and degree of the inflammatory response to bacterial infection and A. xylosoxidans has been shown to be associated with levels of inflammation similar to Pseudomonas aeruginosa in chronically infected CF patients [Reference Ronne-Hansen5].
Studies documenting the pathogenic properties of Achromobacter spp. are scarce and relatively little is known of the mechanisms which promote survival, colonization and progression to infection of the CF lung. In general, it is believed that the capability of bacteria to initiate and persist in chronic infections is due to their biofilm-forming ability, which renders them tolerant towards antimicrobial agents and host defence mechanisms [Reference Bjarnsholt6, Reference Tom7]. In addition, acquisition of antibiotic resistance and bacterial motility have been repeatedly cited as interdependent mechanisms which favour persistence of bacteria in host tissues [Reference Molin and Tolker-Nielsen8–Reference Boles and Horswill11].
On sequencing the genome of the A. xylosoxidans NH44784-1996 strain, Jakobsen et al. [Reference Jakobsen12] identified the presence of an operon encoding an adhesin which had earlier been implicated in biofilm development by Escherichia coli [Reference Wang, Preston and Romeo13]. Despite this suggestion of a mechanism that could promote the persistence of A. xylosoxidans in the CF airway, few studies have addressed the ability of Achromobacter CF isolates to form biofilms [Reference Trancassini14, Reference Abbott and Peleg15].
We have previously reported a high prevalence (21·8%) of airway colonization/infection by A. xylosoxidans in 179 patients treated in two CF centres in Brazil, and that more than half of the patients were classed as having chronic infection [Reference Pereira16]. Isolates were genetically heterogeneous but chronicity was associated with a relatively few of several clones identified by pulsed-field gel electrophoresis (PFGE). We also found evidence of cross-infection with the same clone in over half of all A. xylosoxidans-positive patients [Reference Pereira16].
A. xylosoxidans is the type species of the Achromobacter genus which comprises 15 named species and multiple genogroups (http://www.bacterio.cict.fr/). Recently, new laboratory technologies have allowed the recognition of species other than A. xylosoxidans in CF patients, with A. ruhlandii being identified as the second most prevalent Achromobacter spp. in the United States [Reference Spilker, Vandamme and Lipuma17], but by contrast, A. ruhlandii has rarely been reported from CF patients in Europe [Reference Coward18, Reference Amoureux19].
In this study we investigated the distribution of A. xylosoxidans and A. ruhlandii in Achromobacter spp. recovered from patients treated in CF centres in Rio de Janeiro, as well as the presence of virulence traits associated with bacterial colonization of the host respiratory mucosa, such as biofilm formation, bacterial mobility and antimicrobial resistance.
METHODS
A total of 122 archived Achromobacter spp. isolates recovered over a 5-year period from 39 patients treated in two Brazilian CF centres were included in the study. All isolates had been recovered from respiratory secretions and grouped into 22 clonal types defined by PFGE [Reference Pereira16].
Isolates were identified to species level by amplification and sequencing of gene bla OXA-114-like, and by multilocus sequence type (MLST) as described previously [Reference Spilker, Vandamme and Lipuma20]. Allelic profiles and sequence types (STs) were assigned according to the PubMLST website (http://pubmlst.org/achromobacter/).
The minimum inhibitory concentrations (MICs) of ceftazidime, ciprofloxacin, imipenem and trimethoprim/sulphamethoxazole were determined with E-test strips (AB Biodisk, Sweden) using recommended CLSI breakpoints for non-Enterobacteriaceae [21]. E. coli ATCC 25922, and P. aeruginosa ATCC 27 853 were used as quality control strains for each run of MIC determination.
Approximately 107 c.f.u. of each isolate in 200 μl Mueller–Hinton broth supplemented with 0·75% glucose were inoculated into three wells of 96-well microplates. After 20 h at 35 ± 2 °C under mild agitation, the wells were washed three times with distilled water to remove non-adherent bacteria and the biofilms were stained with 200 μl of 0·1% Violet Crystal solution for 15 min, washed, and air dried for 2 h. The biofilm-bound stain was released with 200 μl of 95% ethanol and the optical density (OD) at 595 nm of the obtained solutions was determined with a microplate spectrophotometric reader. Microplate wells without bacteria served as negative controls.
To assess the swimming and swarming abilities of isolates, 3 μl of a fresh nutrient broth (Oxoid, UK) culture were spotted onto the centre of plates containing 0·3% (w/v) nutrient agar, or 0·8% (w/v) nutrient broth with 0·5% (w/v) agar containing 0·5% (w/v) glucose [Reference Rashid and Kornberg22]. After 24 h at 30 ± 1 °C, the diameters of the bacterial concentric growths from the inoculation site were measured.
Data were analysed with Graph Pad Prism v. 5·0 (GraphPad Software, USA). Because two or more isolates were recovered from some individuals, we utilized median values of OD595 (biofilm) and ring diameters (swimming and swarming), of the different isolates from each patient. Statistical significance (P < 0·05) was determined by the Mann–Whitney test or by Fisher's exact test to compare antimicrobial susceptibility data.
RESULTS
Amplification and sequencing of the bla OXA-114-like gene and MLST of all 122 Achromobacter isolates identified 28 (23·0%) isolates as A. ruhlandii and the remaining 94 (77·0%), as A. xylosoxidans. Five PFGE clonal groups were identified in 22 A. ruhlandii isolates and 17 PFGE clonal groups in the 94 A. xylosoxidans isolates. Seven (18·4%) of the 39 patients harboured A. ruhlandii alone and 30 (76·9%) grew only A. xylosoxidans; the remaining patient yielded both Achromobacter species. Four (13·3%) of the 30 patients who harboured exclusively A. xylosoxidans isolates developed chronic infection which was defined as the presence of at least three positive cultures for Achromobacter spp. during a 1-year period, with a minimum 1-month interval between cultures; chronic infection was also noted in four of the seven patients harbouring A. ruhlandii.
The predominant ‘outbreak’ clone G previously defined by PFGE [Reference Pereira16] was recovered from 22 patients and all were identified as A. xylosoxidans.
MLST analysis identified 13 STs in 106 isolates; 10 STs in A. xylosoxidans isolates (n = 78) and three STs in A. ruhlandii isolates (n = 28). We could not determine STs in 16 A. xylosoxidans isolates [PFGE clonal group F (n = 11), J (n = 2), N (n = 2), and S (n = 1)]. Three isolates of PFGE clonal group A were further discriminated by MLST: ST13 (n = 2), and ST198 (n = 1). All clone G isolates fell in ST200.
Most isolates of both species were susceptible to the four antimicrobials tested and no significant differences were found in susceptibility between the species to imipenem, sulphamethoxazole/trimethoprim, and ceftazidime but A. ruhlandii isolates were significantly less susceptible to ciprofloxacin (P = 0·002, Table 1). Moreover, although A. ruhlandii isolates from chronic infection also showed a similar susceptibility to three antimicrobials compared to sporadic isolates, there was a strong tendency for the former group towards resistance to ciprofloxacin (P = 0·057). The outbreak clone of A. xylosoxidans exhibited significantly more resistance to ceftazidime than other isolates (P = 0·009, Table 1) and there was a strong tendency towards resistance to imipenem for A. xylosoxidans isolates from chronic infection (P = 0·054).
Table 1. Distribution, MIC and rates of susceptibility to four antimicrobials in Achromobacter spp. recovered from cystic fibrosis patients
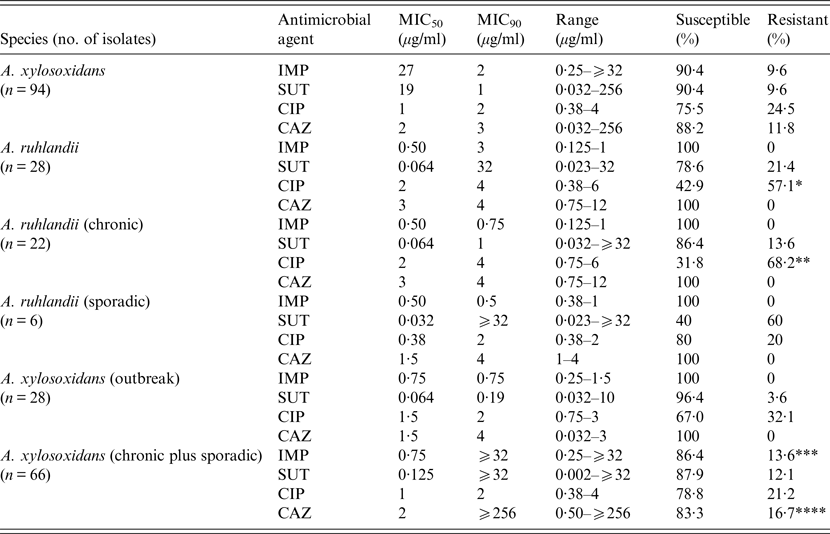
MIC, Minimum inhibitory concentration; IMP, imipenem; SUT, sulphamethoxazole/trimethoprim; CIP, ciprofloxacin; CAZ, ceftazidime.
*P = 0·002: A. ruhlandii × A. xylosoxidans (CIP); **P = 0·057: A. ruhlandii chronic × A. ruhlandii sporadic (CIP); ***P = 0·054: A. xylosoxidans (chronic plus sporadic × A. xylosoxidans (outbreak) (IMP); ****P = 0·009: A. xylosoxidans (chronic plus sporadic × A. xylosoxidans (outbreak) (CAZ).
All isolates of both species formed biofilms on microplate wells and there was no significant difference in the degree of biofilm formation between A. xylosoxidans and A. ruhlandii [median OD595 (range) = 0·910 (0·310–1·858) and 0·667 (0·300–1·500), respectively]. Similarly, for A. xylosoxidans, isolates from chronic or sporadic infection exhibited comparable biofilm-forming capacity [median OD595 (range) = 0·858 (0·684–0·944) and 0·915 (0·300–1·858), respectively], as well as isolates of the outbreak clone compared to either chronic or sporadic infection [median OD595 (range) = 1·013 (0·693–1·217) and 0·858 (0·310–1·858), respectively]. Further, no significant difference in biofilm-forming capacity was evident for A. ruhlandii isolates from chronic or sporadic infection (median OD595 (range) = 0·895 (0·300–1·500) and 0·591 (0·545–1·106), respectively All isolates of both species showed a swimming phenotype but 4·3% and 44·4% of A. xylosoxidans and A. ruhlandii, respectively, were negative in tests for the swarming phenotype. Figure 1 shows that the swimming and swarming abilities of A. xylosoxidans were significantly higher than those of A. ruhlandii. Similarly, the mobility of isolates of the A. xylosoxidans outbreak clone was significantly higher than for other isolates of this species.

Fig. 1. (a) Swimming and (b) swarming motility of Achromobacter xylosoxidans and A. ruhlandii. (c) Swimming and (d) swarming motility of A. xylosoxidans isolates of the outbreak clone and patients with chronic or sporadic infection. Data are medians, and bars represent the interquartile range. * P < 0·05, *** P < 0·001.
DISCUSSION
Prevalence rates of Achromobacter spp. recovered from CF respiratory secretions have increased in recent years. This may be due to the generally extended lifespan of these patients and the selective pressure of prolonged and multiple antimicrobial therapy. Similarly, the prevalence may be simply a consequence of increased ascertainment due to the use of improved microbiological isolation and molecular identification techniques [Reference Lipuma23] which have allowed the recognition of species other than A. xylosoxidans in the CF airway.
In an attempt to understand better the epidemiology, prevalence and level of expression of virulence traits, and antibiotic susceptibility of Achromobacter spp., 122 archived isolates from 398 patients attending two CF centers in Rio de Janeiro [Reference Pereira16] were further investigated. Spilker et al. [Reference Spilker, Vandamme and Lipuma17] have shown through nrdA gene sequences of isolates recovered from 341 CF patients that A. ruhlandii accounted for 23·5% of all Achromobacter isolates, a proportion markedly similar to our finding of 23·0% in the isolates reported here, and represented 21·0% of our patient cohort. Interestingly, over half (57·1%) of the patients harbouring A. ruhlandii isolates developed chronic infection compared to 13·3% in patients who grew A. xylosoxidans alone. However, Staphylococcus aureus and P. aeruginosa were isolated sporadically from three patients, but in two (who were siblings), A. ruhlandii of the same ST was the sole organism isolated before death. Although not statistically significant owing to the small number of patients studied, our findings possibly suggest that patients infected/colonized by A. ruhlandii are at a higher risk of developing chronic infection. It is noteworthy that a clone of Achromobacter spp. designated the Danish epidemic strain which was reported to have chronically infected 13 patients from two Danish CF centres [Reference Ronne-Hansen4, Reference Ridderberg24], was subsequently identified as A. ruhlandii [Reference Ridderberg, Wang and Nørskov-Lauritsen25]. Moreover, the species has also been implicated in causing cross-infection between CF patients even after limited and indirect contact between them [Reference Hansen26], and further supports the view regarding its possible concern in CF communities.
Achromobacter spp. are intrinsically resistant to many antimicrobials agents, and the development of acquired resistance is common during the course of chronic infection, especially for β-lactams [Reference Wang27, Reference Hu28]. Moreover, variability in antimicrobial resistance of CF Achromobacter strains has been reported, although almost exclusively for A. xylosoxidans [Reference Trancassini14, Reference Lambiase29]. Nevertheless, most Achromobacter isolates from our collection proved susceptible to all four antibiotics tested; the agents were chosen according to the recommendation of the CF Consensus Study Group [Reference Doring30]. No significant difference in susceptibility was noted between species and infection status except for the outbreak clone of A. xylosoxidans which showed significantly increased resistance to imipenem and ceftazidime.
Adaptive mechanisms of bacteria probably explain why relatively few pathogens are able to survive and persist in the respiratory tract of CF patients despite an augmented host defence and intensive antibiotic therapy. It is well known that P. aeruginosa, and several other species are able to adapt to the CF host lung environment by switching to a biofilm mode of growth [Reference Ciofu31] and this property is highly correlated with the establishment of chronic infections in these patients. This present study has shown that all A. xylosoxidans and A. ruhlandii isolates produced large, and similar amounts of biofilm on an abiotic plastic surface. Although biofilm formation by A. xylosoxidans from CF patients has been previously reported, both in vivo [Reference Hansen32] and in vitro [Reference Trancassini14], our study extends this property to A. ruhlandii.
Horizontal exchange of genetic material in bacteria occurs with enhanced efficiency within biofilms [Reference Molin and Tolker-Nielsen8], where the dense population structure promotes plasmid dispersal through conjugation which may stimulate biofilm development. Released DNA stabilizes biofilm structure and mediates genetic exchange through transformation. Since A. xylosoxidans can serve as a reservoir of horizontal genetic transfer elements commonly involved in spreading antibiotic resistance [Reference Traglia33], the biofilm mode of growth is likely to favour the development of multidrug resistance in CF Achromobacter.
Swimming and swarming are flagella-dependent types of bacterial motility in low-viscosity liquid and viscous environments, respectively. Most A. xylosoxidans and A. ruhlandii isolates studied here were shown to exhibit the swarming motility phenotype. This is in contrast to the findings of Trancassini et al. [Reference Trancassini14] who were unable to detect a swarming phenotype in 57 strains of A. xylosoxidans. Swarming has been proposed to contribute to bacterial virulence as it facilitates movement of the bacteria through the mucous layer of host epithelia and is considered to be an important means of surface epithelial colonization [Reference Partridge and Harshey34]. In some species, such as P. aeruginosa, swarming cells were shown to exhibit overexpression of a large number of virulence-related genes [Reference Overhage35], whereas bacterial mutants with altered swarming motility were also defective in biofilm formation [Reference Overhage35, Reference Shrout36]. Therefore, swarming is likely to be involved in early biofilm development. Finally, bacterial cells under swarming conditions have also been shown to be more resistant to the action of antibiotics than their non-swarming counterparts due to a type of adaptive resistance, and not the result of mutant selection [Reference Overhage35, Reference Kim37, Reference Lai, Tremblay and Deziel38]. This is particularly important because like other types of adaptive resistance, swarming would contribute to antimicrobial therapy failure even if the strains appeared susceptible by routine in vitro analysis [Reference Partridge and Harshey34]. In this study, A. xylosoxidans isolates, and particularly of the outbreak clone, proved to be better swarmers than chronic or sporadic isolates. This also held true for isolates of A. ruhlandii. It is therefore tempting to speculate that swarming mobility may be a key determinant of the ability of strains to spread through cross-infection between patients, but further research in this area is clearly warranted.
In conclusion, this investigation has provided some novel and interesting data on the relative prevalence of A. xylosoxidans and A. ruhlandii, in CF patients and the susceptibility of such isolates to antimicrobials commonly used for the treatment of these patients in our centre. Although biofilm formation and mobility mechanisms were largely similar in the isolates, an outbreak strain of A. xylosoxidans exhibited enhanced swarming ability and there was some correlation between this and isolates from chronic as opposed to sporadic infections. We believe that our study has given us better insight into the infectious disease process of Achromobacter in the CF lung and further investigations to clarify the relationship between the expression of bacterial virulence traits and ability to colonize/infect CF patients are justified.
ACKNOWLEDGEMENTS
The study was approved by the Research Ethics Committee of State University of Rio de Janeiro under number CAAE: 00716512.0.3001·5269. It was supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; grant no. E-110·742/2012 and the Conselho Nacional de Desenvolvimento Científico e Tecnólogico (CNPq; grant no. 471326/2012-7), Brazil.
We thank Marcia Jones for technical support.
DECLARATION OF INTEREST
None.





