INTRODUCTION
Coxiella burnetii is a small Gram-negative intracellular pathogen belonging to the γ subgroup of proteobacteria [Reference Raoult and Marrie1]. It is the causative agent of the zoonosis Q fever, first described as an outbreak of undiagnosed febrile illness in abattoir workers in Australia in 1935 [Reference Maurin and Raoult2]. The acute presentation of disease is protean and may be suspected based on demographic and epidemiological risk factors. Acute illness may manifest as pneumonia, hepatitis or meningoencephalitis, although the predominance of disease manifestations has been noted to vary depending on geography [Reference Raoult and Marrie1]. Q fever may also develop into a chronic infection, classically as endocarditis.
In Atlantic Canada, the first cases of Q fever were identified in Nova Scotia in 1979 [Reference Marrie3]. In this region, pneumonia remains the dominant form of acute Q fever and interestingly, the major risk factor for acquisition has been exposure to infected parturient cats or newborn kittens [Reference Kosatsky4–Reference Langley7]. Seroepidemiological studies in Nova Scotia have shown that in addition to cats, cattle have served as a dominant reservoir in domestic animals with a low seroprevalence in sheep and goats [Reference Marrie8]. Antibodies to C. burnetii have also been identified in Nova Scotian wildlife including snowshoe hare, moose and raccoon populations [Reference Marrie, Embil and Yates9]. Despite awareness of Q fever in the province, Q fever secondary to exposure to sheep has not been previously recognized in Nova Scotia. In the present study we describe the first case of Q fever in Nova Scotia, acquired as a result of direct exposure to sheep. A follow-up seroprevalence study in the associated flock, suggests that C. burnetii has now spread to a more traditional animal reservoir in Nova Scotia.
Case report
From February to May 2006, over 60 lambs were born in a flock of almost 150 sheep owned and cared for by a 56-year-old farmer and his wife in rural Nova Scotia. The farm is mixed, generating a small amount of produce as well as being residence to one horse, one donkey and two dogs. There are no cats on the farm. The farmer was previously healthy, although a heavy smoker, smoking 1–2 packs per day over the past 30 years. His wife has a history of mild rheumatoid arthritis, treated with hydroxychloroquine. Both were actively involved in the lambing of the parturient ewes. The wife routinely delivered the lambs while the husband physically stabilized the ewes during the birthing. It was noted in April 2006 that one ewe (E1) had been unwell during the lambing. This ewe gave birth to one stillborn lamb in addition to a second that died immediately following birth. Subsequently within hours, the ewe also died. The farmer cleaned out the stall and birthing contents and further lambing that season took place in the same stall.
In early May, two more ewes (E2 and E3) were lambed. It was later noted that these two ewes had shared a stall with E1. E2 delivered three lambs on 1 May, two of which died after delivery. E3 delivered two healthy lambs on 5 May. Two weeks later, the farmer noted significant fatigue. The following day he developed fever and chills with a non-productive cough. On 24 May, he presented to the local emergency department with a fever of 40°C. His white blood cell count measured 15·4×109/l with an absolute neutrophil count of 12·3×109/l. He had a mild hypo-osmolar hyponatraemia (serum sodium 132 mmol/l) with normal renal and liver function. A chest radiograph, shown in Figure 1, revealed a rounded opacity in the superior segment of the right lower lobe measuring 6 cm in diameter. He was diagnosed with community-acquired pneumonia (CAP) and treated as an outpatient with 7 days of levofloxacin. Near completion of the antimicrobial therapy, he noted feeling improved.

Fig. 1. Chest radiograph from 24 May 2006 showing a round pulmonary infiltrate.
Because of persistent opacificaiton on a follow-up CT scan, 15 days after presentation (Fig. 2), he was referred to a respirologist to rule out the possibility of neoplasm. The respirologist, felt the clinical history was in keeping with CAP. Furthermore, with the history of exposure to parturient sheep elicited, serological testing for Q fever was arranged using serum collected on 16 June 2006. Testing showed the farmer had IgG antibodies to C. burnetii by enzyme-linked immunosorbent assay (ELISA), although was negative for IgM antibodies. Indirect immunofluorescence (IFA) testing measuring IgA, IgG and IgM antibodies to C. burnetii revealed phase I antibody titres measuring 1:32 and phase II antibody titres of 1:512 consistent with acute Q fever (Table 1).
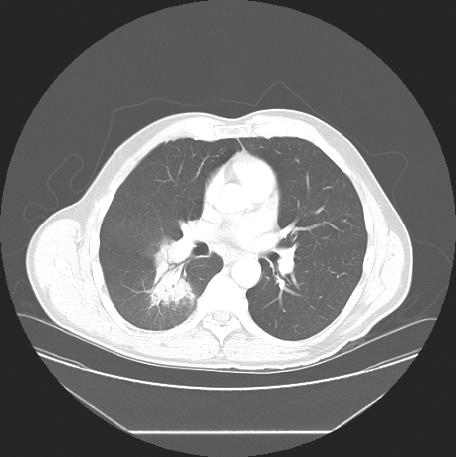
Fig. 2. CT chest scan from 8 June showing persistence of the pulmonary infiltrate.
Table 1. Q fever serology and indirect immunofluorescence testing of Nova Scotia sheep farmer with Q fever pneumonia
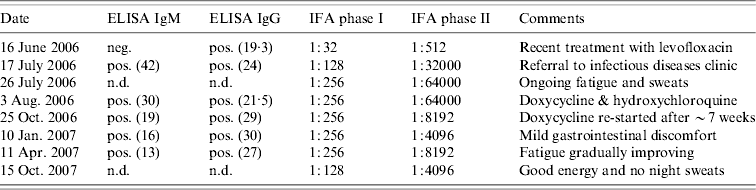
ELISA, Enzyme-linked immunosorbent assay; IFA, immunofluorescence assay; n.d., Not done; neg., negative; pos., positive.
Numbers in parentheses refer to the optical density reading.
A follow-up chest radiograph on 17 July confirmed resolution of the infiltrate. However, repeat serology by ELISA was notable for IgM seroconversion and an increase in the phase I and phase II titres by IFA to 1:128 and 1:32000 respectively. Subsequently, the farmer was referred to an infectious diseases specialist.
Upon assessment at the infectious diseases clinic on 3 August, the farmer was noted to be in general good health, however, he described ongoing fatigue, occasional sweats with intermittent lower extremity myalgias, although no fevers or other constitutional or respiratory symptoms. On physical examination his vital signs were normal and bibasilar inspiratory crackles that cleared with cough were noted on pulmonary auscultation. The remainder of the examination was unremarkable. There were no cardiac murmurs and no stigmata of endocarditis or liver disease. Given the ongoing fatigue and rising titres, there was a concern of evolving chronic Q fever. A transoesophageal echocardiogram was arranged and treatment with doxycycline 100 mg p.o. b.i.d. in conjunction with hydroxychloroquine 200 mg p.o. t.i.d. was recommended. Repeat serology at this visit revealed the phase I titre had increased to 1:256 and the phase II titre to 1:64000 by IFA. Interestingly, his wife was found to have negative Q fever serology.
Because this was the first known case of human C. burnetii infection in Nova Scotia associated with exposure to sheep, a serosurvey of the farmer's flock was undertaken.
METHODS
Serological testing for C. burnetii infection
Humans
ELISA
Serology for antibodies to C. burnetii was performed at the Virology & Immunology Laboratory at the QEII Health Sciences Centre in Halifax, Nova Scotia using commercially available IgM and IgG ELISAs (Panbio Diagnostics, Brisbane, Australia). Testing was performed according to the manufacturer's instructions. The IgM ELISA includes an IgG absorption step that removes competing IgG antibody and nullifies the effect of rheumatoid factor reducing the possibility of a false-positive result. Index values (defined as sample absorbance/calibrator cut-off value) of >1·1 were considered positive and values between 0·9 and 1·1 were considered indeterminate.
IFA
Tests for antibodies to C. burnetii phase I and phase II antigens were performed by IFA at the QEII Health Sciences Centre as previously described [Reference Embil, Williams and Marrie10]. All references to the IFA test in this study refer to this methodology, measuring total IgA, IgG and IgM antibody titres unless otherwise specified. Briefly, slides were fixed with phase I and phase II C. burnetii antigens obtained from the Nine Mile strain and subsequently overlaid with sera initially diluted 1:8 with phosphate-buffered saline (PBS) followed by serial doubling dilutions. Slides were incubated for 30 min at 37°C, washed with PBS and then air-dried. Slides were then overlaid with anti-human IgG conjugated with fluorescein isothiocyanate (FITC) and incubated for 30 min at 37°C in the dark followed by further washing with PBS and distilled H2O. Slides were assessed for fluorescence at 40× power and titres determined.
Sheep serosurvey
Blood samples were collected by external jugular venepuncture of 46 sheep on the farm on 17 August 2006. Specimens were obtained predominantly from ewes and lambs. Samples were kept frozen at −20°C pending analysis. Testing for phase I and phase II C. burnetii antibodies was performed by IFA as described above. Titres were determined using FITC-conjugated rabbit anti-sheep IgG (Dako Diagnostics Canada Inc., Ontario, Canada).
Polymerase chain reaction (PCR) for C. burnetii on sheep milk
Fresh milk was obtained from four nursing ewes (E2, E3, E4 and E5) on the same day blood samples were collected. Aliquots of milk were kept frozen at −70°C and later thawed for PCR analysis. Briefly, DNA was obtained from milk specimens using a Qiagen DNA extraction kit (Qiagen, Mississauga, Ontario). Amplification of the C. burnetii was attempted using a previously described protocol targeting the superoxide dismutase gene [Reference Stein and Raoult11]. The primers employed were a pair of 20- and 19-residue oligonucleotide primers [primer C.B.-1 (5′-ACT CAA CGC ACT GGA ACC GC-3′) and primer C.B.-2 (5′-TAG CTG AAG CCA ATT CGC C-3′)]. Amplification products were resolved by electrophoresis with a 12% polyacrylamide gel followed by ethidium bromide staining and UV illumination for assessment of the 257 bp amplicon.
RESULTS
While 23 of 46 sheep (50%) were seropositive by IFA for the phase II antigen, only a single ewe (2·2%) had antibodies to the phase I antigen (Table 2). This ewe (E3), also had the study's highest titre (1:128) to the phase II antigen.
Table 2. Distribution of antibody titres to phase I and phase II C. burnetii antigens in 46 sheep belonging to a flock associated with a case of Q fever in Nova Scotia
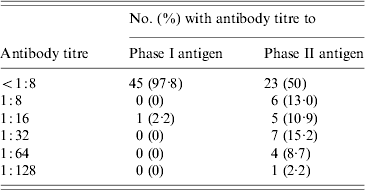
Four sheep (8·7%) had phase II titres of 1:64 including ewe E2 and two other nursing ewes, E4 and E5. The latter two ewes had pregnancies that overlapped with E2 and E3. E4 and E5 had each delivered single healthy lambs on 1 and 4 July respectively. E2 and E3 had not been in the same pen as E4 and E5 while they were gravid, however, they did later share a stall for nursing purposes.
Seven sheep (15·2%) had phase II titres of 1:32. Four of these were lambs that were nursing from E2, E3, E4 or E5 at the time of the study. However, molecular studies failed to detect C. burnetii DNA in any of the milk specimens obtained from these four ewes.
Patient follow-up
Following his first month of therapy, the farmer was mistakenly dispensed only hydroxychloroquine without doxycycline. As such, he was essentially off treatment for 7 weeks at the time of follow-up at the infectious diseases clinic on 25 October 2006. At that time, he described feeling better overall, however, he continued to experience persistent fatigue with occasional night sweats and feverish sensations. His physical examination was unchanged and his phase I titre remained stable at 1:256 while his phase II titre had begun to decrease (Table 1).
Treatment with doxycycline and hydroxychloroquine was re-instituted and on further follow-up, he had tolerated these medications with only mild gastrointestinal discomfort. A transoesophageal echocardiogram on 15 November 2006 identified no abnormalities. His energy has slowly improved and his night sweats have gradually resolved.
The subsequent lambing season on the farm began December 2006. By mid-April 2007, there had been in excess of 100 lambs born. One ewe that had been ill prior to lambing had to be euthanized at the time of delivery and, despite a subsequent rapid caesarean section, the two lambs in utero were stillborn. In addition there were other miscarriages and stillborn lambs involving otherwise healthy ewes. Following counselling on occupational exposures and prevention, the farmer now disposes of the birthing products by incineration rather than composting. The farmer's wife has undergone repeat Q fever serological testing and remains negative to date. As such, use of a mask with lambing and when cleaning birthing products was advised as vaccine was not available for administration.
DISCUSSION
Following the 1935 Q fever outbreak in Queensland, Australia, it was concluded that domestic animals were the secondary reservoir of Q fever. Wild animals were identified as the natural reservoir with transmission among and within reservoirs occurring via ticks and other arthropods [Reference Maurin and Raoult2]. The zoonosis has since been identified on a global scale. In cases of human Q fever, the most commonly identified reservoirs of C. burnetii, the causative pathogen, include sheep, goats, cattle and cats [Reference Marrie12]. The natural history of Q fever in sheep is such that active disease is not observed clinically. The exceptions to this otherwise asymptomatic state in sheep are the pathological manifestations of abortion and stillbirth associated with chronic infection [Reference Maurin and Raoult2]. As the bacteria has a tropism for the uterus and mammary glands, the placenta and birthing products may be heavily contaminated. As such, shedding of the organism occurs mainly during parturition. Additionally, C. burnetii has been recovered from the milk of sheep [Reference Maurin and Raoult2].
There are two major points of interest in this present report. First, this is the first Nova Scotia case of ovine-associated Q fever and the sheep flock is noted to have high seroprevalence. Second, this case report highlights the shortcomings of the serological diagnosis of Q fever. These points will be the focus of this discussion.
There have been many reports of Q fever outbreaks and high incidence of disease in areas with high sheep densities [Reference Dupuis13–Reference Spicer15]. In addition, human infections have occurred in research and laboratory facilities through exposure to infected sheep [Reference Hall, Richmond and Caul16–Reference Rauch19]. Exposure to airborne particles and farming are important risk factors. A 1995 cohort study of five English local authority districts found that C. burnetii seroprevalance increased significantly with exposure to the farm environment (P<0·01) [Reference Thomas20]. A statewide study conducted in North Dakota following a case of Q fever in a 37-year-old sheep farmer found that in 496 sheep producers, family members and hired helpers, there was a significantly increased risk of Q fever infection associated with assisting with lambing particularly in those with physical contact with the sheep during the lambing process (OR 6·4, P=0·04) [Reference Guo21].
There is also data to suggest that C. burnetii may be transmitted from one animal reservoir to another. Goats were identified as the source of a Q fever outbreak in Newfoundland in 1999 [Reference Hatchette22]. Subsequent to this, a study of domestic ruminants in that province noted a significant increase in the rates of C. burnetii seroprevalance in the sheep populations [Reference Hatchette23]. The seropositivity had increased from 3·1% in 1997 to 23·5% in 1999–2000 (P<0·001). It is speculated that the increasing seroprevalence in sheep was related to the goat-associated Q fever outbreak, although conclusive evidence was not obtained.
Accordingly, it is of interest to speculate on the potential introduction of C. burnetii into the sheep flock described in this Nova Scotia study. A number of the sheep had been purchased from other owners in recent years, and it is possible that some of these sheep were infected at the time of importation with subsequent spread to other sheep within the flock. Other possibilities include transmission from the wildlife reservoir or from other domestic reservoirs.
More than 40 tick species are known to be naturally infected with C. burnetii and believed to be important in maintaining the lifecycle of C. burnetii in the natural environment and may transfer infection to domestic animals [Reference Maurin and Raoult2, Reference Marrie12]. Data from Cyprus has shown that a systemic programme to control ticks in the flocks significantly reduced abortions due to Q fever in sheep and goats [Reference Polydorou24]. In our case, the farmer had noted ticks on many of his sheep in the months preceding his infection. Furthermore, some existing data demonstrates that Nova Scotia ticks may also act as vectors for Q fever. Organisms obtained from Dermacentor variabilis and Haemophilis leporus-palustris ticks collected in Nova Scotia in 1980 were found to be positive by direct fluorescence microscopy to sera against C. burnetii (unpublished data of Max Garvie, provided by Harvey Artsob via written communication 21 August 2006).
Cats as the primary source of C. burnetii with subsequent transmission to the flock must also be considered. Although there were no cats on this farm, a large number of the sheep in this study were purchased from another farm in the area. The barn in which these sheep had been kept was also occupied by a large number of barn cats. Given the previously documented high seroprevalence rate in cats in the province of Nova Scotia and the relatively lower rate in sheep in the province, this raises the question of whether the cats had been the original reservoir in this case [Reference Kosatsky4–Reference Marrie6, Reference Marrie8]. Unfortunately sera from these cats were unavailable for testing.
With some of the flock infected, recurrent environmental exposure to C. burnetii probably played an important role in contributing to a high seroprevalance in the flock as a whole. Contamination of the environment with infected milk or direct transmission to lambs is one possibility. Four nursing lambs were identified with phase II titres of 1:32. However, molecular analysis of the milk obtained from the ewes did not detect C. burnetii. Nonetheless, intermittent shedding cannot be excluded, and milk may have played a role, although previous studies do suggest lower rates of C. burnetii in sheep milk as compared to the milk of goats and cattle [Reference Maurin and Raoult2, Reference Kim25, Reference Muramatsu26].
A high established concentration of C. burnetii in the environment is probably the largest factor contributing to exposures and high seroprevalance in the flock. Contamination of the environment from products of conception will occur given the high bacterial load in these tissues. Persistence in the environment is facilitated by the organism's ability to resist extremes of temperature and desiccation. Much of this resistance may be attributed to the extracellular stability of metabolically dormant small-cell variants (SCV). These SCVs undergo vegetative differentiation into more metabolically active large-cell variants following phagocytosis by eukaryotic cells [Reference Heinzen, Hackstadt and Samuel27]. C. burnetii spore-like particles have also been observed and these forms are also hypothesized to contribute to the organism's resistant nature.
Furthermore, the lifecycle of C. burnetii includes antigenic phase variation with observed changes in the lipopolysaccharides (LPS) [Reference Hackstadt28]. These mutational variations have implications for virulence, entry into eukaryotic cells and survival as well as Q fever diagnostic strategies. The virulent phase I variant, corresponding to smooth LPS, is highly infectious and is found in infected animals, including arthropods and humans. In contrast, phase II variants correspond with rough LPS and are avirulent. This latter phase variant may be obtained through serial passage in cell culture or embryonated egg cultures. Phase I C. burnetii are poorly internalized by monocytes and macrophages, although they are able to survive within these cells. In contrast, the phase II forms readily gain entry into these cells, however, they are quickly killed by the phagolysosomal pathway [Reference Mege29]. It is also interesting to note that although phase I organisms are the infectious variant, the immune response to acute C. burnetii infection is characterized by an early rise in the total phase II antibody titre by complement fixation (CF) that generally peaks at 3 months and persists at moderate levels beyond 1 year, rather than the phase I antibodies which are indicative of chronic Q fever [Reference Dupuis30]. This difference may be a result of the phase I antigen being less immunogenic.
The human serological response to C. burnetii and its phase variants is complex and deserves some focus in this discussion given the unusual serological response noted in this case. Antibodies to C. burnetii can be determined by a variety of tests including microagglutination, CF, IFA, and ELISA [Reference Fiset31–Reference Peter34]. ELISA methods are good qualitative measures of Q fever serology but the optical densities generated often do not correlate with absolute antibody titres and they cannot differentiate between antibodies to phase I and phase II antigens that is necessary to differentiate acute from chronic infection. In contrast, other methodologies may allow for measurement of titre responses to phase I and phase II antigens that may be used to help classify the infective state. For example, using CF, a high antibody titre to the phase I antigen of >1:200 has been considered compatible with chronic Q fever or Q fever endocarditis [Reference Leedom, Remmington and Schwartz35].
IFA is the test commonly used by reference laboratories to obtain phase I and phase II titres. The French National Reference Centre for Rickettsial Diseases in Marseille, France performs serological testing by IFA with the Nine Mile strain using an initial serum 1:25 dilution with serial twofold dilutions. Employing this methodology, the value of titre combinations was assessed at the National Reference Centre in Marseille, using the paired sera of 2218 individuals with known clinical evolution. It was found that a phase II IgG titre ⩾1:200 in combination with a phase II IgM titre ⩾1:50 is highly predictive of evolving acute or chronic infection with a specificity of 100% [Reference Dupont, Thirion and Raoult36]. This same study also found a phase I IgG antibody titre of ⩾1:800 highly predictive of and sensitive for chronic Q fever with a specificity of 99·6% and a sensitivity of 100%. This latter serological finding has been found to be valuable in establishing the diagnosis of infective endocarditis and has been added as a major criterion within the modified Duke criteria [Reference Li37, Reference Raoult38].
A recent retrospective serological analysis of 22 patients with Q fever endocarditis provides further evidence that following acute Q fever, regular serological monitoring may be warranted in order to identify individuals at high risk of developing endocarditis. The findings of this study, from the National Reference Centre in Marseille, suggest that an increase in the levels of phase I IgG antibodies to titres ⩾1:800 warrants aggressive work-up to rule out endocarditis [Reference Landais39].
However, at present there is no universally accepted standardized method for conducting IFAs and therefore difficulty exists in interpreting test results from assays that use different methods. In this present case study, the IFA employed identifies IgA, IgG and IgM antibodies to C. burnetii. The IFA methodology uses an initial 1:8 dilution of patient sera in PBS with subsequent serial twofold dilutions. A fourfold rise in the convalescent phase II titre or a single phase II titre ⩾1:64 is deemed consistent with acute Q fever in the appropriate clinical setting. An anti-phase I antibody titre ⩾1:256 is considered worrisome for chronic Q fever. As such, this case further demonstrates the potential shortcomings in the serological definition of chronic Q fever. Although the patient in this case study had a phase I antibody titre ⩾1:256, the phase II antibody titre is so much higher than the phase I titre that it is doubtful that this represents chronic Q fever.
There is data to suggest that the ratio of phase I to phase II antibodies in cases of Q fever can be used to distinguish acute from chronic Q fever [Reference Peacock33]. Peacock et al. compared the serological parameters of 15 patients with Q fever using microagglutination, CF and IFA. It was noted that phase II to phase I antibody ratios of >1, ⩾1 and ⩽1 were consistent with primary Q fever, granulomatous hepatic Q fever and Q fever endocarditis, respectively. Applying this serological evaluation in conjunction with the clinical facts of our case study, the data suggests that the farmer's serology is consistent with primary or acute infection rather than chronic disease.
Therefore, while close follow-up and repeat serological testing will be important, it appears unlikely that the farmer in this case has developed chronic Q fever. The heterogeneity of the immune response has been well described [Reference Embil, Williams and Marrie10]. However, the degree of elevation of the farmer's phase II antibody titres is striking. Certainly there are factors related to the bacterial strain in any given exposure, as well as host factors that also impact on an individual's serological response. This combination of factors is further highlighted in this case report when one considers that the wife remained seronegative with repeat testing despite multiple high-risk exposures. Whether the hydroxychloroquine she was receiving for her rheumatoid arthritis has some antibacterial effect is not yet clear and this may have influenced her outcome.
CONCLUSION
In this study, we describe, the first case of Q fever in Nova Scotia, acquired as a result of direct exposure to sheep. Follow-up seroprevalence studies in the associated flock, suggest that C. burnetii has now spread to more traditional animal reservoirs in Nova Scotia. The case is also of interest, given the unusual serological response of the farmer with extremely high phase II antibody titres. In addition, the wife's lack of serological response and disease development despite repeated high-risk exposures is notable.
ACKNOWLEDGEMENTS
The authors thank Dr Chris Cox of Cornwallis Veterinary Clinic, Kentville, Nova Scotia for assistance with obtaining the sheep sera, and Dr Colm McParland of the Division of Respirology, QEII Health Sciences Centre, Halifax, Nova Scotia for assistance with the case management and relevant documentation. Thanks are also due to Dr Harvey Artsob for information regarding C. burnetii infection in Nova Scotia ticks.
DECLARATION OF INTEREST
None.






