Introduction
Shiga toxin-producing Escherichia coli (STEC) are a group of toxin-producing bacteria capable of causing gastrointestinal illness in humans. Of particular importance and the most common serogroup in Scotland is E. coli O157 [Reference Browning1]. The infectious dose required to cause illness is low, with <1000 cells sufficient [Reference Tuttle2, Reference Strachan3]. Clinical presentation ranges from asymptomatic infection to mild non-bloody diarrhoea, through bloody diarrhoea and haemorrhagic colitis to haemolytic uraemic syndrome.
STEC can colonise the gastrointestinal tract of wild, farmed and domesticated animals and be shed in their faeces. Cattle are considered to be the most important reservoir for STEC infection in humans; however, other ruminants such as sheep, goats and deer can also act as reservoirs of infection [Reference Beutin4–Reference Rounds6]. Transmission to humans can occur as a result of direct contact with STEC-contaminated faecal material, such as through handling and petting animals, or indirectly by exposure to faecally contaminated vegetation or by the consumption of contaminated water or food.
Meat may be contaminated with STEC during the slaughter process. Faecal contamination of carcasses by the producer animal is the most important source of STEC strains in meat products from farm animals [Reference Heuvelink7]. The majority of the meat-related outbreaks are due to beef products, in particular undercooked beef burgers [Reference Tuttle2, Reference Bell8, Reference King9]. Less common are outbreaks associated with other meats including pork [Reference Trotz-Williams10], lamb [Reference Sekse11] and venison [Reference Keene12]. STEC infection associated with venison has tended to be sporadic cases [Reference Rabatsky-Ehr13], family clusters or small outbreaks related to the consumption of individually shot and prepared deer carcases [Reference Rounds6, Reference Keene12] rather than commercially produced venison products. In 2015, Health Protection Scotland (HPS) and Food Standards Scotland (FSS) led the investigation into a Scotland-wide outbreak of E. coli O157 Phage Type (PT) 32, which identified commercially prepared venison products as the most likely source; this was the first outbreak in the UK linked to commercially produced venison products. This paper describes the outbreak investigation, measures taken reduce the chance of a similar outbreak occurring in the future and wider work to improve understanding of STEC in venison.
Materials and methods
Outbreak identification
On 30 September 2015, HPS was informed by the Scottish E. coli O157/STEC Reference Laboratory (SERL) of six cases of E. coli O157 PT 32 with an indistinguishable multi locus variable number tandem repeat analysis (MLVA) profile (8-5-6-14-6-6-10-13). The cases were resident in multiple NHS Board areas across Scotland. Scotland is divided into 14 NHS Board areas, each responsible for the local public health management of STEC cases. The identification of the outbreak resulted in the formation of a national Incident Management Team (IMT).
Case interviews
As part of the routine response to STEC infections in Scotland, NHS Board Health Protection Teams interview all reported cases. These interviews are conducted on the same day as reported (or as soon as possible thereafter). In 2015, these interviews were conducted using locally developed enteric disease questionnaires, which capture information on clinical presentation and a range of exposures, including travel, animal contact and a food history. HPS obtained copies of the completed enteric questionnaires for all cases, a review of these questionnaires did not identify a single common link between the cases. However, it was noted that of the first four questionnaires from primary cases, three had reported the consumption of venison. To help identify any common links, four of the initial cases were re-interviewed as soon as possible after the identification of the outbreak using a longer and more in-depth trawling questionnaire previously developed by the Public Health England (PHE) for use in outbreak investigations. The trawling questionnaire captures information on a wide range of exposures in the 7 days prior to the onset of symptoms including travel, events or functions attended, recreational and outdoor exposures, contact with animals, as well as a very detailed food history for a wide range of foods eaten both within the home and outside. The initial trawling questionnaires and information from some of the enteric surveillance forms identified a number of common food vehicles, in particular, venison, beef mince and vegetables. Based on these findings, a more focused questionnaire was developed that collected more detailed information in relation to the purchase, preparation and consumption of a number of commonly reported items (venison, beef mince and vegetables). This more focused questionnaire was used to interview the other new cases as they were identified and identified venison as the common product reported by cases, and in particular, a possible link to venison products produced by a single food business operator (FBO).
Close contacts of confirmed cases were screened where indicated in line with guidance for the management of STEC in Scotland [14] to identify any secondary cases.
Human microbiology
In Scotland, faecal samples from symptomatic individuals are submitted to local diagnostic laboratories for culture, and presumptive isolates of E. coli O157 are forwarded to the SERL for confirmation and typing. Faeces testing negative at the local diagnostic laboratory but from individuals with symptoms suggestive of a STEC infection, or from symptomatic contacts of known cases, are also forwarded to SERL for more sensitive testing for all STEC in line with current Scottish guidance [14].
At the time of the outbreak, all E. coli O157 isolates were sub-typed using phage typing [Reference Ahmed15] and MLVA [Reference Holmes16].
In order to compare E. coli O157 isolates in Scotland with isolates from cases in England and Wales, SERL sent Scottish isolates to the PHE Gastrointestinal Bacteria Reference Unit (GBRU) for whole-genome sequencing (WGS). For WGS, DNA was extracted by PHE from the cultures of E. coli O157 for sequencing on the Illumina HiSeq 2500 instrument as described previously in the work of Jenkins et al. [Reference Jenkins17].
Food traceback and environmental investigations
Once a possible link to venison products produced by a single FBO was identified, FSS initiated investigations at the FBO including an on-site inspection. The inspection reviewed processing methods, storage facilities and the FBOs food safety management system, based on the legal requirements for Hazard Analysis and Critical Control Points (HACCP) procedures. In addition, FSS assessed the procedures for ensuring product traceability and carried out a programme of microbiological sampling.
Food and environmental microbiology
Food samples were taken from the FBO premises, products manufactured by the FBO were collected from retail outlets (n = 8) and a sample of venison sausages produced by the FBO was collected from the home freezer of one of the cases. This product had been opened by the case but was produced at the same time as the implicated batches. A number of environmental swabs were also taken at the FBO. All food and environmental samples were processed at the local public analyst laboratory and potential isolates of STEC sent to SERL for confirmation and typing.
Results
Case definitions
Cases must have been resident in Scotland for part or all of the 7 days before onset of symptoms and have a date of onset on or after 11 September 2015. This was the first onset date among the cases and there was no evidence of this MLVA being identified in Scotland prior to that date.
Confirmed case
An individual with confirmed infection with E. coli O157 PT32 of the outbreak MLVA profile.
or
Secondary case
A confirmed case with onset 2 or more days after another confirmed case that is a household or other close contact.
Confirmed cases
Twelve confirmed cases were identified as E. coli O157 PT32 (stx1 negative, stx2 positive) with the outbreak MLVA profile. SERL had commenced routine MLVA typing of all E. coli O157 isolates in December 2012 and had a database of approximately 850 isolates. The outbreak MLVA profile was unique and not previously identified in the SERL database. WGS of the isolates from Scotland did not identify any cases with the same WGS profile in England and Wales.
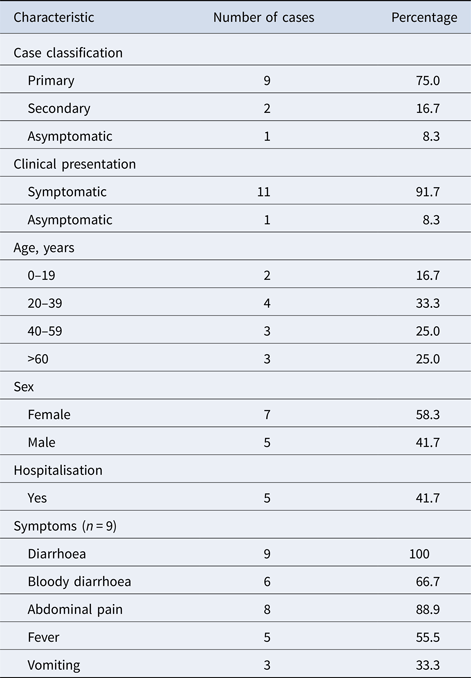
Among the 12 cases with the outbreak MLVA profile, nine were primary cases, two secondary cases and one asymptomatic case (Table 1).
Table 1. Characteristics of Escherichia coli O157 PT32 case with outbreak MLVA profile (n = 12)
Demographics and clinical characteristics
The cases were aged between 3 and 81 years (mean 40 years, median 41 years).
The cases lived across Scotland. There was one household cluster comprising one primary, two secondary and one asymptomatic case.
Dates of onset ranged from 11 September 2015 to 17 October 2015. The last case (date of onset 17 October) consumed venison products purchased during the same time frame as the other cases; however, these had been frozen following purchase and eaten at a later date (Fig. 1). Of the remaining eight primary cases, onset dates ranged from 11 September to 30 September 2015.

Fig. 1. Date of onset for primary and secondary cases of Escherichia coli O157 PT32 with outbreak MLVA and key dates in investigation (n = 11).
The first case had an onset date of 11 September and the outbreak was identified 19 days later on 30 September with six cases. This time difference is due to the time required for the cases to seek medical attention, submit a stool sample, the identification of E. coli O157 at the diagnostic laboratory, the submission of the samples to SERL and the MLVA results becoming available.
Food exposures
Focused or trawling questionnaires were completed for eight of the nine primary cases. The ninth case was identified and interviewed after the outbreak was declared over and interviewed using the local NHS Board's routine enteric questionnaire with additional follow-up questions in relation to venison consumption. Analysis of the supermarket loyalty card for this case revealed that they had purchased venison products produced by the same FBO during the same time period as the other cases. All nine of the primary cases reported having consumed and/or handled venison products in the 7 days prior to onset of symptoms. These had been purchased raw and cooked in the home. Of the nine cases, eight had consumed venison, and one case had not eaten venison themselves but had handled and cooked raw venison in the household. Eight of the nine primary cases reported that the venison products were from ranges manufactured by a single FBO (Table 2). Of these, five reported consuming products made from wild venison from a particular range that was only sold in Scotland.
Table 2. Venison products consumed or handled by primary cases (n = 9)
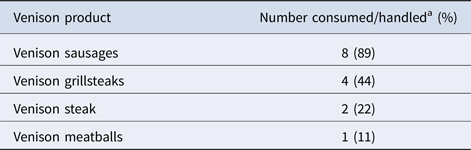
a Some cases reported eating more than one product.
No other food vehicle was identified that accounted for the same high proportion of cases as venison. Whilst the initial enteric questionnaires and trawling questionnaires had also identified some other foods of interest, on completion of the focused questionnaire none of these foods apart from venison were reported by all cases. Additionally, no commonality was identified for the brand or place of purchase for the other food vehicles.
Since 2009, PHE have operated an enhanced surveillance scheme for STEC infection in England whereby data, including food exposures, are collected from all reported cases using a standardised questionnaire. In order to place the consumption of venison by primary cases in this outbreak into a wider context, PHE interrogated their STEC-enhanced surveillance database. Of approximately 6000 STEC cases reported between 2009 and 2015, only 30 (0.005%) reported the consumption of other ‘other meat, e.g. game’; the category under which venison would be recorded. Whilst recognised that there could be some under ascertainment of venison consumption through the enhanced surveillance dataset, it provided additional evidence of the rate of venison consumption reported by cases in this outbreak being much higher than expected. This is further supported by information subsequently obtained on the consumption of venison in the general population from the Food Standards Agency from the National Diet and Nutrition Survey (2008/09–2011/12) [18], which estimated that 0.6% of 3371 consumers (19 years and older) had consumed venison in the previous 4 days.
Food and environmental microbiology
All food samples tested negative for E. coli O157. Non-O157 STEC strains of serotype O130:H11 and O113:H17 were isolated from four samples.
All environmental swabs taken at the FBO were negative, except for samples taken from the cutting table and its associated drain. These showed the presence of E. coli O157; however, this was of a different phage type than the outbreak strain (PT8 stx1 and stx2 positive). This was not an entirely unexpected finding as these samples were taken from areas where raw meat was being processed. Further swabs taken following deep cleaning of the premises showed an absence of STEC. No human isolates were identified by SERL that matched the isolates obtained from the food samples or the environmental swabs. The fact that the outbreak strain was not isolated from the FBO was not too surprising as the samples were taken several weeks after the production of the implicated products and the plant would have undergone daily cleaning during that time.
Risk management
By 3 October, 3 days after initial identification of the outbreak, the IMT had strong evidence linking the outbreak to venison products produced by a single FBO. The most likely hypothesis was that cases became infected through either cross-contamination from, or undercooking of contaminated raw venison product in the home. Based on purchase and consumption dates provided by cases and manufacturing dates supplied by the FBO, FSS were able to identify the product ranges, batches and use-by dates most likely implicated in the outbreak. It was determined that, given the use-by dates, these products would no longer be on sale in retail premises. However, it was recognised that consumers may have these batches stored in freezers at home. FSS issued an advice notice informing consumers of the affected products and reinforcing the importance of safe handling and cooking of these products.
The FBO cooperated fully with the investigation, voluntarily suspending production and acting promptly to implement recommended control measures. Production restarted following a series of deep cleans and negative environmental samples taken at the premises. FSS undertook on-site inspection at the FBO to review how the plant processes and produces its products, its storage facilities and HACCP procedures. The on-site inspection found that production hygiene, structure and maintenance were generally of a high standard. However, the systems employed during carcass processing were noted to present a potential cross-contamination risk, and the procedures for the dressing of large wild game were adjusted to minimise future risk. It was ascertained that the FBO had been carrying out fortnightly routine testing of their products in line with regulatory requirements set out in Regulation (EC) 2073/2005 on microbiological criteria for foodstuffs, but that the corrective action to increase sampling to weekly when an unsatisfactory result was obtained had not been followed. The FBO reviewed and changed its procedures and frequency for microbiological sampling in line with guidance provided by FSS. Based on the assumption that wild venison had the potential to present a higher theoretical risk of STEC contamination than farmed products, the FBO reviewed its supply chains and risk factors for the contamination of wild deer meat throughout the process.
During the investigation, it was noted that some raw products were packaged together with sachets of sauce. This was recognised as a possible source of cross-contamination within the domestic kitchen. The FBO now vacuum packs raw meat products and sauces separately to reduce the risk of cross-contamination.
Discussion
This was the first reported outbreak of STEC linked to venison products in the UK; although the outbreak strain was not isolated from the products tested, there was a strong epidemiological evidence linking the consumption of venison products to this outbreak. This outbreak was notable due to the number and national distribution of cases due to the implicated products being a commercially produced and widely distributed product. In contrast, previous venison outbreaks reported mainly from the USA have tended to be smaller and related to individually prepared carcasses [Reference Rounds6, Reference Keene12, Reference Rabatsky-Ehr13].
There are no published estimates of the prevalence of STEC in either farmed or wild deer in Scotland. Estimates from elsewhere have shown a wide range, influenced by different deer species, microbiological testing protocols and wildlife–livestock interactions [Reference Singh19], but with generally low rates for E. coli O157. Escherichia coli O157 was isolated from 0.25% of post-slaughter faecal samples in one study from the USA [Reference Renter20], similar to 0.3% and 0.6% from two other US-based studies [Reference Dunn21, Reference Fischer22]. Rates for all STEC have generally been higher but still wide ranging. For example, in one study from Spain, E. coli O157 was detected in just 0.5% of samples from roe deer, whilst non-O157 STEC was detected in 52.5% [Reference Mora23]. A second study from Spain reported E. coli O157 in 1.5% of red deer faecal samples and non-O157 in 34% [Reference Diaz-Sanchez24], whilst in Belgium, STEC was detected in 12.0% of faecal samples, none of which were O157 [Reference Bardiau25]. Similarly, a further study from Japan isolated STEC from 16.3% of wild deer samples, none were O157 [Reference Asakura26] and a study in Switzerland detected stx and eae genes in 21.4% and 18.8% of hunted wild red and roe deer, respectively [Reference Obwegeser27]. The detection of non-O157 in food samples tested during this outbreak investigation fits with these studies showing the carriage of a range of non-O157 strains in deer at higher frequencies than O157 strains.
In the European Union, wild game and game meat are covered by the food hygiene legislation (Reg (EC) No. 852/2004 and 853/2004 (EU Food Hygiene Regulations)), but there is currently no requirement in the UK for the FBO to test raw meat for the presence of STEC. As deer are known to carry STEC, the assumption is that there is potential for STEC to be present in raw meat; and whilst there are no published estimates of STEC contamination of raw venison meat in the UK, STEC has been detected in venison at retail sale in the USA [Reference Magwedere28] and Spain [Reference Diaz-Sanchez29]. Therefore, for the products implicated in the outbreak, the application of good hygiene and proper cooking by the consumer were important control measures for managing the STEC risk. The FBO provided validation records for the cooking instructions provided on the packaging of each of their raw venison products. These showed that the cooking instructions were adequate for ensuring these products reached the desired temperature to eliminate pathogens as recommended by the Advisory Committee on the Microbiological Safety of Food (ACMSF). In addition to providing guidelines on cooking times and temperatures (but highlighting that all appliances vary), the advice on all meat preparation packaging states ‘ensure the product is cooked thoroughly and there is no pink meat visible’ as the thorough cooking of the products would have eliminated STEC. It was the view of the IMT that either undercooking and/or cross-contamination within the home were contributing factors in this outbreak. As the majority of cases reported they had thoroughly cooked the products and one case had only handled the products and had not consumed them, it is likely that cross-contamination within the home was an important factor. This leads to the hypothesis that the products attributed to the outbreak could have been made using the same source of heavily contaminated venison, and it is important to consider the potential reasons for such contamination.
The FBO produced venison products using both farmed and wild animals, and products implicated in the outbreak contained wild venison. The contamination of game is usually related to the manner in which the animal is killed, dressed, handled and processed [Reference Rabatsky-Ehr13]. This can be more difficult to control for wild deer, which are slaughtered and eviscerated (gralloched) in the field, and can therefore be exposed to STEC contamination from the environment.
In cattle, some animals are known to be ‘supershedders’ excreting high levels of STEC [Reference Arthur30]. Whilst it is not known if the same is true for deer, the occurrence of any such animals may help explain why one batch of venison products could be particularly heavily contaminated.
It may also be of relevance that the outbreak occurred in September, as it is widely recognised that shedding of E. coli O157 in cattle has a seasonal dependency. Recent research has shown that herd-level prevalence of STEC in Scotland was significantly lower in spring than autumn [Reference Henry31]. It therefore seems reasonable to assume that higher levels of STEC are circulating in the environment during this period, which would increase the potential for carcass contamination. There is much less evidence of seasonal shedding patterns in deer, and although a study from the USA showed that STEC was more likely to be detected in deer in June compared with March [Reference Singh19], a longitudinal study of farmed deer did not show a seasonal trend in shedding of E. coli O157 [Reference Dunn21].
The consumption of venison in the UK has increased in recent years, with estimates by the Scottish Venison Partnership of around a 10% increase year on year, and on this basis, UK consumption of venison will rise from 3800 to more than 6000 tonnes by 2021 [32]. At least in part of this increase is due to a positive comparison with beef, with a lower intramuscular fat content and higher polyunsaturated/saturated fatty acid ratio [Reference Bures33]. The increasing consumption trends for venison make learning from this incident especially important. This outbreak has highlighted some important knowledge gaps in relation to STEC in venison. Scottish Government and FSS are currently funding new research that is supported by the Scottish venison industry to address some of these gaps; first, sampling of deer faeces for STEC in different geographical areas, to explore seasonality and impact of co-grazing with ruminants. Second, the sampling of carcases for generic E. coli at different stages from being shot in the field to the Approved Game Handling Establishment to ascertain where the highest chance of cross-contamination may occur.
The FBO and the wider venison industry in Scotland have also been very proactive in learning from this incident and introducing measures to reduce the risk of similar outbreaks in the future and participating in research to identify factors associated with STEC contamination of venison and factors that may decrease the chance of cross-contamination during production.
The outbreak has also highlighted the role of cross-contamination within the domestic kitchen and the importance of consumer educational campaigns to raise awareness of the steps to reduce cross-contamination when handling raw meat products.
Acknowledgements
The authors would like to express their thanks to all members of the Incident Management Team and are grateful to the patients for their cooperation during the investigation.
Collaborators
Incident Management Team: G. Hawkins, S. Ahmed, L. Browning, A. Smith-Palmer, A. Reynolds, L. Kidd, J. Schofield (Health Protection Scotland), R. Bruce, B. Campbell, J. McElhiney, M. Tocher (Food Standards Scotland), M. Hanson, L. Allison (Scottish E. coli O157/STEC Reference Laboratory), B. Cullinane (NHS Ayrshire and Arran), B. Lawrie (South Ayrshire Council), P. Scoular, C. Sumpter, H. Prempeh (NHS Forth Valley), S. Fisher, L. McGilvary (Stirling Council), H. Henderson (Clackmannshire Council), D. Webster, L. Byers (NHS Grampian), J. Strachan, L. Wellington (NHS Lothian), L. Denvir, D. Chandler, T. McMichael, H. Paul, E. Baird (NHS Tayside), M. Quinn (NHS Fife), D. McCormick (Scottish Government Health Protection Team), R. Elson, C. Jenkins (Public Health England).








