Introduction
Louse-borne relapsing fever (LBRF) is a classic epidemic disease, associated with war, famine, refugees, poverty, crowding and poor personal hygiene. After a long history, recorded over many centuries, it is now largely confined to the Horn of Africa, while retaining its potential to cause future epidemics when conditions become conducive. It was a familiar infection in Europe and North America until the end of the 19th century after which it was forgotten. However, the recent surge of refugees from Africa arriving in European countries has brought this fascinating disease back into the view of the medical profession and has stimulated new research into its cause, Borrelia recurrentis, and its vector, the human body louse.
Aetiology [Reference Felsenfeld1]
LBRF is caused by B. recurrentis, a large, loosely coiled, motile spirochaete (family Spirochaetaceae, that also includes Treponema), with tapering ends, 12–22 μm long and 0.2–0.6 μm thick, with an average wavelength of 1.8 μm, an amplitude of 0.8 μm and 8–10 periplasmic flagella [Reference Cutler2]. They divide by transverse binary fission. B. recurrentis can be cultured on chick chorioallantoic membrane, and maintained in rodents [Reference Felsenfeld1]. Strains of immunodeficient mice (SCID lacking B and T cells, and SCID BEIGE lacking B, T, and NK cells) have been proposed as an animal model of LBRF [Reference Larsson3]. B. recurrentis can be cultured in vitro using Barbour-Stoenner-Kelly (BSK-II) medium [Reference Cutler4], BSK-H supplemented with heat-inactivated 10% rabbit serum and modified-Kelly-Pettenkofer (MKP) medium supplemented with 50% fetal calf serum [Reference Marosevic5]. BSK medium supports rapid initial borrelial growth but this is followed by cell deformation and death, whereas MKP medium appears to improve isolation rate, morphology and motility [Reference Ružić-Sabljić6].
Unlike other bacteria, borreliae have a fragmented genome consisting of a linear chromosome, 1–15 linear plasmids and 1–9 circular plasmids. B. recurrentis has the simplest genome of all, composed of one linear chromosome and only seven linear plasmids, and only 990 protein coding genes. It shows low genetic variability [Reference Marosevic5]. Genomes of B. recurrentis and B. duttonii are identical except that in B. recurrentis 30 genes or gene families of B. duttonii are either absent or damaged. This has been cited as evidence that B. recurrentis has a decaying genome and is only a strain or subset of B. duttonii that adapted rapidly to louse-transmission with genome reduction [Reference Lescot7]. B. recurrentis lacks RecA and RadA proteins that are responsible for DNA repair. The average nucleotide identity between the African borreliae, B. crocidurae, B. duttonii and B. recurrentis, is 99%, suggesting that they are merely ecotypes of the same genomospecies ‘B. africana’ [Reference Elbir8].
Transmission
Unlike most borreliae, transmission of B. recurrentis is restricted to one vector, the human body louse Pediculus humanus corporis, and, perhaps, the head louse P. humanus capitis. Although B. recurrentis has been identified in head lice, including those infesting pygmies in the Republic of Congo, outside the currently recognised geographical distribution of LBRF [Reference Amanzougaghene9], transmission by them has not yet been confirmed. Body lice, unlike head lice, retreat from the skin after feeding to hide and lay their eggs in clothing seams rather than on hair shafts. In Addis Ababa, one old man was found to be harbouring more than 21 500 lice in his clothes [Reference Sholdt, Holloway and Fronk10]. Lice are obligate haematophagous human ectoparasites that ingest borreliae in their blood meal [Reference Sangaré, Doumbo and Raoult11]. They are intolerant of deviations in human body temperatures caused by fever, climatic exposure or death, or when infested clothing is discarded. Then, they find a new host to whom borreliae can be transmitted. Coelomic fluid from a crushed louse, or louse faeces infected with B. recurrentis, is inoculated through broken skin, or intact mucous membranes such as the conjunctiva, by scratching. Blood transfusion, needlestick injuries and contamination of broken skin by infected blood are potential causes of nosocomial infections [Reference Bryceson12]. Since lice, unlike ticks, cannot infect their progeny, they do not act as reservoirs. Transplacental infection has been confirmed in a mouse model of B. duttonii infection [Reference Larsson13] and there are reports of congenital infection by B. hermsii and other tick-borne spirochaetes [Reference Fuchs and Oyama14]. There is no known animal reservoir, and so persistence of infection between epidemics can only be through mild or asymptomatic human infections.
Epidemiology and historical background
Human disasters created by war, forced migrations, poverty, famine, breakdown of personal hygiene and seasonal spells of cold, wet weather, promote crowding and increase the risk of infestation by body lice and the transmission of LBRF, louse-borne typhus, trench fever and other louse-borne diseases. LBRF can be identified in historical descriptions of disease epidemics by the repeated recurrences of fever between asymptomatic periods of 4–7 days and by two typical symptoms, jaundice and bleeding. The earliest convincing description of this disease was given by Hippocrates in the 5th century BC in the North Aegean island of Thasos: ‘The great majority (of sufferers) had a crisis on the sixth day, with an intermission of six days followed by a crisis on the fifth day after the relapse.’ Other features typical of LBRF were severe rigors, jaundice, profuse epistaxes and tendency to precipitate abortion [Reference Lloyd15, Reference MacArthur16]. MacArthur has argued convincingly that the ‘Yellow Plague’ that engulfed Europe in 550 AD, in the wake of the Justinian plague, and the famine fevers of the 17th and 18th centuries in Ireland and elsewhere, whose defining feature was jaundice, were predominantly LBRF [Reference MacArthur16].
Recently, a historical genome of B. recurrentis was recovered from the skeleton of a young woman found during the excavation of a graveyard near St. Nicolay's Church in Oslo. Radiocarbon dating suggested that its age was AD 1430–1465. The mediaeval European genome displayed an ancestral oppA-1 gene, and gene loss in antigenic variation sites (variable short and long membrane protein genes) that translated into a genome reduction of 1.2% of the pan-genome, and 5.1–21% of the affected plasmids, perhaps associated with increased virulence but a reduced number of relapses [Reference Guellil17].
In Dublin in 1770, Rutty described ‘a fever altogether without the malignity attending (typhus), of six or seven days duration, terminating in a critical sweat…in this the patients were subject to a relapse, even to a third or fourth time, and yet recovered’ [Reference Rutty18]. In Edinburgh in 1843, Craigie distinguished LBRF from typhus and coined the name ‘relapsing fever’ [Reference Craigie19]. Henderson detailed the differences between the two infections [Reference Henderson20]. In Britain, in the 19th century, LBRF featured prominently in Charles Murchison's treatise on continued fevers. He commented on its intermittent appearance and truly epidemic nature: ‘So completely did relapsing fever disappear from Britain after 1828 that when, after an interval of fourteen years, it again showed itself as an epidemic in 1843, the junior members of the profession failed to recognize it and it was regarded as a new disease’ [Reference Murchison21]. Obermeier saw spirochaetes, now recognised as B. recurrentis, in the blood of febrile patients in Berlin in 1866 [Reference Wright and Maria22]. Transmission by human body lice was proved by Mackie in 1907 [Reference Mackie23].
In the 20th century, from 1903 to 1936, a huge pandemic swept across North Africa, the Middle East and Africa, causing an estimated 50 million cases with 10% mortality. A second epidemic in 1943–46 created 10 million cases [Reference Sangaré, Doumbo and Raoult11].
An endemic focus persists in the Horn of Africa [Reference Sparrow24]. In cold, wet weather, impoverished people with louse-infested clothes crowd together for warmth and shelter. These indigent, malnourished street-dwellers, day workers (casual labourers), usually young men and prisoners, are the most vulnerable to infection. In the Ethiopian highlands there are annual epidemics of thousands of cases coinciding with the rains. Outbreaks have also occurred in Somalia. In Rumbek County, South Sudan, in 1999–2000, there were 20 000 cases with some 2000 deaths, 580 in January 1999 alone [25]. In 1985, in Chavin District if Ancash Province in the Peruvian Andes at altitudes above 3800 m, 60 clinical cases were reported among louse-infested villagers, 36 with B. recurrentis in their blood films [Reference Valdizan, Lopez and Delgado26]. More recently in Calca Province in the Urubamba Valley of Peru, antibodies to B. recurrentis have been found in two of 194 villagers [Reference Raoult27]. The discovery of B. recurrentis in head lice in Congolese pygmies raises the possibility of other undiscovered human reservoirs [Reference Amanzougaghene9].
B. recurrentis infection in African refugees arriving in Europe
Since July 2015, LBRF has been diagnosed in almost 100 mainly young male refugees, who arrived in several European countries, most in Italy and Germany, seeking asylum after travelling from Ethiopia, Eritrea, Somalia and other African countries, usually through Libya [Reference Ciervo28–Reference Isenring34]. It is the most frequently reported infection in Eritrean immigrants [Reference Isenring34]. It seems likely that many other cases may have gone undetected and unreported [30]. Most of the patients were from the Horn of Africa, but the duration of their symptoms suggested that the majority had been infected in Libya. However, two cases diagnosed in Turin, Italy were long-term residents who shared accommodation with recently arrived immigrants, suggesting the possibility of autochthonous infection [Reference Antinori31]. Some, from African countries not endemic for LBRF such as Mali, were probably infected during their journey, in crowded transit hostels in Libya, Italy or elsewhere [Reference Grecchi35]. LBRF was largely unknown in Germany and other European countries before its re-emergence after 2015 [Reference Hoch29], but there is now the possibility that it might become re-established in some impoverished and crowded immigrant populations in parts of Europe [Reference Antinori31].
Pathophysiology and pathology
The relapse phenomenon
Attacks of relapsing fever end abruptly when specific bactericidal immunoglobulin M antibodies generated by the B1b cell subset lyse spirochaetes in the blood, independently of complement and T cells. Between relapses, spirochaetes may persist extracellularly in spleen, liver, kidneys, eye and other sites. Relapses, accompanied by spirochaetaemia, are explained by antigenic variation, which has been studied in depth in the North American tick-borne pathogen, B. hermsii [Reference Barbour and Hayes36]. Silent gene sequences from an archive stored in plasmids are transposed to one end of an expression linear plasmid where their recombination leads to synthesis of a new variable major outer membrane lipoprotein (vmp) [Reference Barbour and Hayes36]. The new external membrane allows borreliae to evade the host's humoral immune response until antibodies are generated against the new serotypic vmp antigen, explaining the sequential emergence of borreliae expressing different vmps during the course of an untreated infection. Stimulation of the massive release of tumour necrosis factorα (TNF-α) at the start of the Jarisch–Herxheimer reaction (J-HR) to antibiotic treatment in LBRF is also due to vmp [Reference Vidal37]. B. recurrentis is protected from the host's innate immunity by expressing receptors that selectively bind C4bp and C1-Inh, the endogenous regulators of the classical and lectin complement pathway, HcpA protein that, by binding plasmin, decreases C3b deposition and a specific receptor for the serum-derived complement inhibitor of the alternative pathway, CFH [Reference Grosskinsky38]. These mechanisms allow evasion of lysis by complement activation. Another possible protective mechanism is rosetting of erythrocytes around some Borrelia spirochaetes that masks or excludes them by steric hindrance, from host antibody. However, although B. crocidurae and B. duttonii induce rosetting, B. recurrentis do not [Reference Guo39].
Pathophysiology
The spontaneous crisis that terminates untreated attacks, and the J-HR induced by antibiotic treatment, show pathophysiological features of a classic tri-phasic endotoxin reaction. B. recurrentis outer membrane vmps stimulate monocytes to produce TNF-α through NF-κB [Reference Vidal37]. There are transient marked elevations in plasma concentrations of TNF-α, interleukin (IL)-6, IL-8 and IL-1β [Reference Negussie40, Reference Coxon41]. The massive burst of cytokines is triggered by phagocytosis of spirochaetes opsonised by the antibiotic. Penicillin binding results in large surface blebs on the spirochaetes which are then phagocytosed by neutrophils in the blood and by the spleen. In vitro, surface contact with spirochaetes induces mononuclear leucocytes to produce inflammatory cytokines and thromboplastin, causing fever and disseminated intravascular coagulation [Reference Butler42]. Kinins may be released during the J-HR. The profound leucopenia that develops during the reaction reflects sequestration rather than leucocyte destruction. Spirochaetes may be found in liver, spleen, myocardium and brain. Thrombocytopenia rather than vasculitis causes the petechial rash. Cardiorespiratory and metabolic disturbances result from persistent high fever, accentuated by the J-HR or spontaneous crisis [Reference Warrell43].
Pathology [Reference Judge44, Reference Anderson and Zimmerman45]
Spirochaetes are mainly confined to the lumen of blood vessels, but tangled masses occur in splenic miliary abscesses and (Fig. 1C) infarcts as well as within the central nervous system adjacent to haemorrhages. A perivascular histiocytic interstitial myocarditis, found in the majority of fatal cases, may be responsible for conduction defects, arrhythmias and myocardial failure resulting in sudden death. Splenic rupture with massive haemorrhage, cerebral haemorrhage and hepatic failure are other causes of death [Reference Salih46]. There is hepatitis with patchy midzonal haemorrhages and necrosis, meningitis and perisplenitis. Serosal cavities and surfaces of viscera are studded with petechial haemorrhages and sometimes massive pulmonary haemorrhages, reminiscent of leptospirosis. Thrombi are occasionally found occluding small vessels, but the peripheral gangrene, that is a feature of louse-borne typhus (Rickettsia prowazekii infection) [Reference Perine47, has not been reported in LBRF [Reference Bryceson12].
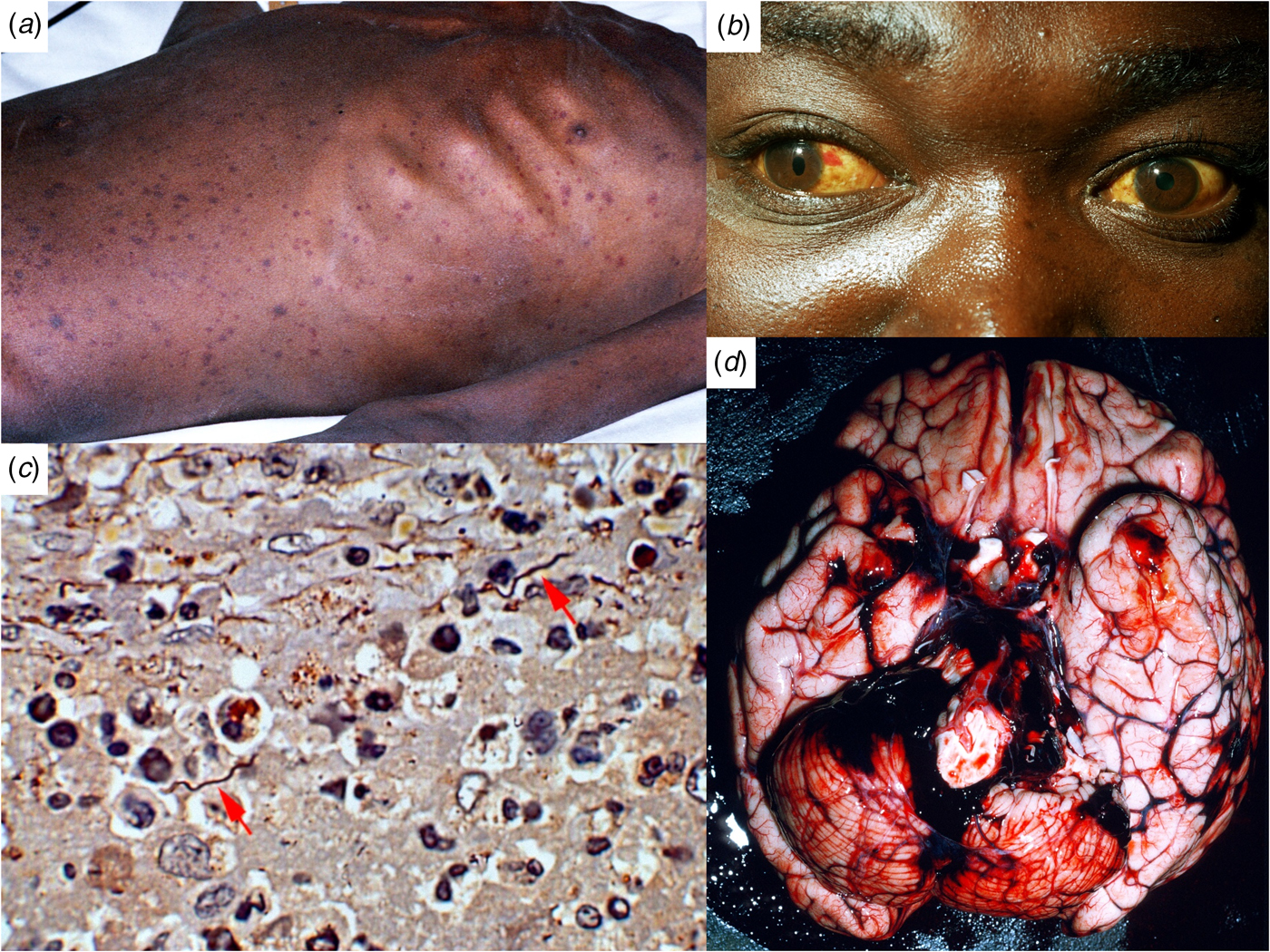
Fig. 1. Ethiopian patients with LBRF. (A) Profuse petechial rash on the trunk in an emaciated patient with complicating infection with Salmonella enterica serovar Typhi (S. Typhi). (B) Subconjunctival haemorrhages and jaundice indicative of hepatocellular damage, thrombocytopenia and coagulopathy. (C) B. recurrentis spirochaetes arrowed (silver stain) in the splenic pulp. (D) Cerebral haemorrhage on the 6th day of illness, a common cause of death in patients with LBRF.
Symptoms and signs [Reference Bryceson12]
The incubation period is 4–18 (average 7) days. The attack starts abruptly with a fever that increases to nearly 40 °C in a few days, accompanied by rigors. Early symptoms include headache, dizziness, nightmares, generalised aches and pains; affecting especially the lower back, knees and elbows; anorexia, nausea, vomiting and diarrhoea. Upper abdominal pain, cough and epistaxis develop later. Prostration and confusion are the rule. The commonest sign is hepatic tenderness (in about 60%) and enlargement (50%). Splenic tenderness and enlargement are less common. Jaundice is found in seven to >70% of patients [Reference Bryceson12]. A petechial or ecchymotic rash, particularly involving the trunk (Fig. 1A), is seen in 2% to 80% of patients [Reference Bryceson12]. It must be distinguished from the maculo-papular or petechial rash of louse-borne typhus. Subconjunctival haemorrhages (Fig. 1B) and epistaxis (25%) are common, haemoptysis, gastrointestinal bleeding and retinal haemorrhages less so. Many patients have myalgia. Meningism occurs in about 40% of patients. Neurological symptoms are less common than in tick-borne relapsing fevers: cranial nerve lesions, monoplegias, flaccid paraplegia and focal convulsions. Untreated attacks resolve by crisis after 4–10 (average 5) days, followed by an afebrile remission of 5–9 days, succeeded by up to five relapses of diminishing severity, during which there may be epistaxis but no petechial rashes.
Pregnant women are especially susceptible to severe disease and premature labour, and still births are frequent. In tick-borne relapsing fever caused by B. duttonii, intrauterine growth retardation, placental damage and inflammation, impaired fetal circulation and maternal anaemia have been described. Spirochaetes frequently cross the placenta, resulting in congenital infections [Reference Larsson13].
Clinical in refugees arriving in Europe
Clinical findings and results of laboratory investigations in 55 refugees [Reference Antinori48] have been compared with those in 62 Ethiopian patients studied in Addis Ababa in the late 1960s [Reference Bryceson12]. Symptoms such as fever, headache, myalgias, abdominal pain and vomiting were common in both groups, but, among the refugees, jaundice was more often reported (51% vs. 34%). Bleeding (8.9% vs. 23%), meningism (5.5% vs. 39%), J-HRs (62% vs. 100%) and fatalities (1.8% vs. 4.8%) were less common. Levels of C-reactive protein [median 284 (55–440.9) mg/dl] and procalcitonin [13.93 (0.95–62.1) ng/ml] were raised in the refugees [Reference Antinori48]. Twenty percent of the refugees had raised serum creatinine concentrations [2.4 (0.9–4.7) mg/dl], indicating renal impairment [Reference Antinori48]. Body lice were recovered from 22% of the refugees and in others there were scratch marks suggesting infestation [Reference Antinori48].
Children: In Sheshamane, Ethiopia, children younger than 15-years-old were compared with adults [Reference Ramos49]. Clinical features in children resembled those in adults but were generally less severe and less frequent. Headache (40%), dizziness (39%), abdominal cramps (17.4%), vomiting (23.8%), cough (27.6%), musculoskeletal pain (30.5%), petechial rash (1.9%) and bleeding (3.8%) were all less common in the children [Reference Ramos49]. In a later study from the same hospital, fever, headache, dizziness and musculoskeletal pains were said to be the commonest symptoms [Reference Ramos50]. A study of infants and children in Arsi Region, Ethiopia, found that the common clinical features were fever (100%), headache (84.5%), chills (74%), abdominal pain (51%), epistaxis (20%), hepatomegaly (26%), splenomegaly (14%), petechial rash (34%) and jaundice (10%). Pneumonia (14%) and central nervous system involvement (10%) were common complications. J-HRs occurred in 61%. Case fatality was 1.9% [Reference Borgnolo51].
Prognosis
Case fatalities between 30% and 70% have been reported in untreated patients during major historic epidemics, but in treated cases, on average, 2–6% will die [Reference Bryceson12]. In an outbreak in Arsi Zone, Ethiopia in 2016, the case-fatality was 13% [Reference Nordmann52]. Reported case fatalities in children range from 1.9% to 5.5%. In one series of 154 children (<15 years) in Ethiopia, overall case fatality rate was 2.4%, less than in adults (13.2%) [Reference Ramos50].
Severe louse-borne relapsing fever
Clinical features associated with a bad prognosis include coma; shock; hyperpyrexia; myocarditis with acute pulmonary oedema [Reference Parry53]; acute respiratory distress syndrome; hepatic failure; ruptured spleen and haemostatic failure from thrombocytopenia liver damage and disseminated intravascular coagulation leading to intracranial (Fig. 1D), massive gastrointestinal, pulmonary or peripartum haemorrhage [Reference Bryceson12, Reference Warrell43, Reference Ramos50]. Complicating co-infections such as dysentery, salmonellosis, typhoid, typhus, tuberculosis, bacterial pneumonia, visceral leishmaniasis and malaria increase mortality [Reference Bryceson12, Reference Anderson and Zimmerman45].
Spontaneous crisis and J-HR
An impending crisis on about the fifth day of the untreated illness, or a J-HR about 1 to 2 h after antibiotic treatment, is signalled by restlessness and apprehension, followed by distressingly intense rigors lasting 10 to 30 min [Reference Bryceson12, Reference Warrell43]. During this chill phase, temperature, respiratory and pulse rates, and blood pressure rise steeply, with associated delirium, gastrointestinal symptoms, cough and limb pains. Fatal hyperpyrexia may occur. The flush phase, characterised by profuse sweating, a fall in blood pressure, and a slow decline in temperature, may last for many hours. During this period, patients may collapse and die if they stand up or may develop progressive and intractable hypotension, especially if they suffer acute myocardial failure attributable to borrelial myocarditis [Reference Parry53]. Treatment with intravenous tetracycline carries the highest risk of provoking a J-HR, reaching 100% in some studies [Reference Bryceson12]. Low-dose or slow-release penicillin causes fewer reactions but may not prevent relapses (see below). In children, J-HRs are less common.
Laboratory investigations [Reference Bryceson12]
Circulating spirochaete densities may exceed 500 000/mm3 of blood. Patients commonly have a moderate normochromic anaemia with neutrophil leucocytosis. The spontaneous crisis and J-HR are marked by leucopenia. Thrombocytopenia is usual and there is a mild coagulopathy (raised prothrombin time and INR) with evidence of increased fibrinolysis (increased fibrinogen degradation products or D-dimer). Raised serum concentrations of aminotransferases, alkaline phosphatase, direct and total bilirubin and low albumin suggest hepatocellular damage. Mild renal impairment is common. The cerebrospinal fluid shows a lymphocyte or neutrophil pleocytosis without detectable spirochaetes.
Electrocardiographical (ECG) evidence of myocarditis includes prolongation of the QTc interval, T-wave abnormalities and ST-segment depression with transient acute right heart strain after the J-HR and various arrhythmias [Reference Parry53]. Chest radiographs are usually clear but may show pulmonary oedema or pneumonic consolidation.
Diagnosis
Microscopy
The possibility of rapid bed-side diagnosis makes LBRF a satisfying disease for the clinician. Thick and thin blood films should be taken while patients are febrile and stained with Giemsa, May-Grünwald Giemsa, Wright, Wright-Giemsa, Field's, or Diff-Quick stains, or examined under dark-field. Positivity thresholds of thin and thick smear blood are respectively estimated at 105 and 104 spirochaetes per millilitre of blood [Reference Hovette54]. A two-stage centrifugation concentration method has been described [Reference Larsson and Bergström55]. Quantitative buffy coat technique (acridine orange) is also possible. The higher and more persistent spirochaetaemia in LBRF makes microscopic diagnosis more reliable than in the other borrelioses. Exflagellating Plasmodium vivax microgametes may be mistaken for spirochaetes (‘pseudoborreliosis’) [Reference Berger and David56], but microfilariae are far too large to cause confusion.
Polymerase chain reaction (PCR)
An important break-through has been the development of a multiplex real-time PCR (MR-TPCR) method, to differentiate the four main Borrelia species in Africa [Reference Elbir57]. It targets the 16S rRNA gene (detecting all four species); glpQ gene (B. croidurae); recN gene (B. duttonii/B. recurrentis) and recC gene (B. hispanica). The assay has a 100% sensitivity and specificity for B. duttonii/B. recurrentis, but could not discriminate between these two species because of their very close genetic and genomic proximity that suggests they may be a single species [Reference Lescot7, Reference Elbir8]. PCR detected 100 copies, proving to be more sensitive than the 103–105 borreliae/mL visible by microscopy. Among infected immigrants to Europe, PCR has proved a valuable method for confirming the species diagnosis of B. recurrentis [Reference Hoch29–Reference Hytönen32], and has detected some microscopy-negative cases [Reference Hoch29, Reference Antinori48]. PCR has been successfully introduced at a point-of-care laboratory in rural Senegal for diagnosis of B. crocidurae infections [Reference Sokhna58].
Serology
Sera from patients with LBRF may give positive reactions with Proteus OXK, OX19 and OX2, which might suggest the diagnosis. False-positive serological responses for syphilis are found in in 5–10% of cases. Serology has generally proved unreliable and non-specific, but it has been improved by the use of the glpQ gene as a recombinant antigen [Reference Porcella59], or monoclonal antibodies (to B. crocidurae) [Reference Fotso Fotso60]. However, serology lacks sufficient specificity, is not commercially available, and may fail to detect acute infections [Reference Porcella59, Reference Fotso Fotso60].
Differential diagnosis
In a febrile patient in or from Africa, who has all the classic features of LBRF – jaundice, petechial rash, epistaxis, hepatosplenomegaly, thrombocytopenia, coagulopathy and elevated serum aminotransferases – severe falciparum malaria is the most urgent differential diagnosis. In the Horn of Africa, yellow fever and other viral haemorrhagic fevers such as Rift Valley Fever and viral hepatitis, rickettsial infections, especially louse-borne typhus which occurs in mixed epidemics with LBRF, must be considered. If there is evidence of acute kidney injury, leptospirosis is more likely. Co-infection of LBRF with leptospirosis was diagnosed by PCR in a refugee from East Africa who arrived in Italy [Reference Cutuli61]. Trench fever (Bartonella quintana), transmitted by lice, can also cause episodic recurrent fever with headache and pains in the shins, but it lacks the bleeding and jaundice of LBRF. In endemic areas, complicating bacterial infections, particularly typhoid, or coinfection with malaria should not be forgotten [Reference Bryceson12, Reference Anderson and Zimmerman45]. In refugees diagnosed in Europe, P. falciparum malaria, sepsis, leptospirosis and meningitis have been cited as leading differential diagnoses [Reference Antinori48].
Treatment
Antibiotics
Complete cure with prevention of relapses is achievable with a single oral dose of 500 mg tetracycline or 500 mg erythromycin stearate. However, vomiting is so common that parenteral treatment is more dependable. A single intravenous dose of 250 mg tetracycline hydrochloride or, for pregnant women and children, a single intravenous dose of 300 mg erythromycin lactobionate (children 10 mg/kg body weight) is effective. In mixed epidemics of LBRF and louse-borne typhus, a single oral dose of 100 mg doxycycline is effective [Reference Perine and Teklu62]. Benzyl penicillin (300 000 units = 80 mg), procaine penicillin with benzyl penicillin (600 000 units) and procaine penicillin with aluminium monostearate (600 000 units), all by intramuscular injection, are often effective but may fail to prevent relapses [Reference Warrell63]. Long-acting preparations clear spirochaetaemia slowly and the J-HR is protracted. Some experienced clinicians prefer to use a low initial dose of penicillin (adult dose, 100 000–400 000 units by intramuscular injection) in severe cases and pregnant women because they believe that the incidence and severity of the J-HRs will be less. In a randomised clinical trial of three regimens of intramuscular procaine penicillin and one of oral tetracycline in Gondar, Ethiopia, the incidence of J-HRs increased with increasing doses of penicillin, from 5.1% with 100 000 units to 31.1% with 400 000 units, and was 46.6% in patients treated with tetracycline [Reference Seboxa and Rahlenbeck64]. Since fatalities (3.3%) were associated with J-HRs, the authors recommended that treatment be initiated with low dose penicillin, despite relapse rates that decreased from 45% to 9.4% from the lowest to highest doses of penicillin. There were no relapses after tetracycline treatment as had been described earlier [Reference Bryceson12]. The combination of penicillin on the first day of treatment, followed by tetracycline on the next day, deserves further study [Reference Salih and Mustafa65]. Chloramphenicol is effective in a single dose of 500 mg by mouth or intravenous injection in adults. It may not be available in some Western countries.
Preventing the J-HR
Antibiotics, such as tetracycline, that rapidly eliminate spirochaetes from the blood and prevent relapses, often induce a severe, and rarely fatal, J-HR. Antibiotic treatment cannot be withheld in view of the high untreated mortality, especially as severe spontaneous crises, that occur in a large proportion of LBRF cases on or after the fifth day of fever, may also prove fatal. There is no conclusive evidence that the apparently milder reaction following slow-release penicillin, compared to tetracycline is any less dangerous [Reference Warrell63, Reference Seboxa and Rahlenbeck64].
Pre-treatment with oral prednisolone may prevent the J-HR of early syphilis, but neither an oral dose of 3 mg/kg prednisolone given 18 h beforehand, nor an infusion of 3.75 mg/kg betamethasone, prevented the J-HR in LBRF [Reference Warrell66]. Hydrocortisone in doses up to 20 mg/kg [Reference Warrell66], paracetamol [Reference Butler42] and pentoxifylline [Reference Remick67] failed to prevent the J-HR. However, meptazinol, an opioid antagonist/agonist, diminished the reaction when given in a dose of 100 mg by intravenous injection [Reference Teklu68, Reference Wright69]. A polyclonal ovine Fab anti-TNF-α antibody infused for 30 min before treatment with intramuscular penicillin, effectively prevented or diminished the J-HR [Reference Fekade70], but, unfortunately, has not been made available for use in the endemic areas. Recombinant human IL-10 was not effective [Reference Cooper71].
Supportive treatment
During spontaneous crisis or J-HR, hyperpyrexia must be actively prevented with antipyretics, vigorous fanning and tepid sponging. The prolonged ensuing flush phase poses dangers of hypotensive shock and postural hypotension and so patients must be nursed lying in bed for at least 24 h after treatment. Most patients are dehydrated and relatively hypovolaemic and adults may need 4 litres or more of isotonic saline intravenously during the first 24 h. Infusion should not be excessive, but carefully monitoring, by observing jugular venous or central venous pressures. Borrelial myocarditis predisposes to acute myocardial failure during the flush phase when there is a demand for a high cardiac output to sustain blood pressure in the face of systemic vasodilatation. Warning signs are symptoms of acute myocardial failure, a rise in central venous pressure above 15 cm H2O, and prolonged ECG QTc interval [Reference Parry53]. One mg of digoxin given intravenously over 5–10 min has proved effective in this situation [Reference Parry, Bryceson and Leithead72]. Diuretics may worsen the circulatory failure by causing relative hypovolaemia in the presence of the intense vasodilatation. Oxygen should be given during the reaction, particularly in severe cases. Patients with prolonged prothrombin times should be treated with vitamin K. Heparin is not effective in controlling coagulopathy and should not be used. Complicating opportunistic infections (typhoid, salmonellosis, bacillary dysentery, tuberculosis, typhus, visceral leishmaniasis, malaria) must be anticipated and treated appropriately.
Prevention and control of epidemics
For B. burgdorferi, an effective vaccine has been developed for dogs, but not yet for humans. However, there has been no interest in developing vaccines against relapsing fever borreliae.
Breaking louse transmission is essential for the control of an epidemic. Infested clothing should be deloused using heat (>60 °C) or washing at 52 °C for 30 min. Patients should be bathed with soap. Lice have developed some degree of resistance to the most commonly used topical pediculicides, including 10% dichloro-diphenyl-trichloroethane, lindane, 1% malathion, 2% temephos, 1% propoxur and 0.5% permethrin [Reference Sangaré, Doumbo and Raoult11]. Head lice should be removed by washed or shaving although their role in LBRF is unproven. Separating infested clothes from wearers for 10 days starves lice to death at any ambient temperature [Reference Barker and Barker73].
Author ORCIDs
David A. Warrell, 0000-0001-7792-1615
Acknowledgements
I am grateful to the many friends and colleagues with whom I studied LBRF in Ethiopia between 1968 and 1994, notably Anthony Bryceson, Eldryd Parry, Helen Pope (Watkins), David J. Wright, Bayu Teklu, Daniel Fekade and Kyle Knox; and the late Charles Leithead, Peter L. Perine and Jemal Abdulkadir.



