INTRODUCTION
Bovine tuberculosis (TB) is an important animal health issue in Ireland. A national programme of TB eradication commenced in 1954, to address concerns relating to public health and international trade, and initial progress was rapid. However, since the mid-1960s, disease incidence has remained stable and progress towards eradication has stalled [Reference More1]. In recent years, constraints to eradication are increasingly understood, as a result of detailed research conducted in Ireland and elsewhere. To limit disease transmission, under the national programme, all cattle in all herds are tested annually, test-positive animals are removed and these herds restricted from trading until two clear consecutive retests are subsequently achieved.
The role of the badger, has been examined in several large field trials, including the east Offaly project (EOP, during 1989–1995) [Reference Ó2–Reference Ó4]; and the four area project (FAP, 1997–2002) [Reference Griffin5, Reference Griffin6] in Ireland and the Randomized Badger Culling Trial (RBCT, 1998–2006) [Reference Donnelly7–Reference Donnelly12] in the United Kingdom. In each of these studies, levels of bovine TB in cattle were lower in areas subject to extensive proactive badger culling compared to matched reference areas where culling was either very limited (EOP, FAP) or not undertaken (RBCT). Results regarding localized reactive badger culling are less clear. In the RBCT, an increase in TB prevalence in cattle was reported in association with reactive culling [Reference Donnelly7–Reference Donnelly9] and in areas adjacent to proactive culled areas. However, a recent update [Reference Donnelly12] reported that the detrimental effect in the adjacent areas may diminish on successive annual proactive culls. Adverse effects from reactive culling were not observed in the FAP in Ireland [Reference Griffin5, Reference Griffin6].
Wildlife has been identified as an important TB reservoir in a range of countries, including New Zealand [Reference Ryan13], South Africa [Reference Michel14] and the United States [Reference O'Brien15]. In each of these countries, the eradication of TB from cattle populations has proved problematic. The challenges facing Ireland and the United Kingdom are substantial, noting that badgers are a protected and valued national species. In the short-to-medium term, badger culling forms part of the overall Irish control policy. In the longer term, there is a substantial research effort towards the development of an effective TB vaccine for badgers [Reference Gormley and Collins16].
In this paper, we describe an observational study to critically evaluate the long-term effectiveness of proactive badger culling and to gather insights into the long-term effects of reactive culling, on TB prevalence in cattle. We use data from the Irish midlands since 1989, of badger removal and TB incidence in cattle. The data include, but are broader than, the formal study area and period of the EOP [Reference Eves3].
MATERIALS AND METHODS
The observational study
The observational area
Observations were conducted over an area that included, but was larger than, the EOP study areas. These included an ‘inner removal area’ (in earlier publications termed ‘project area’ 528 km2), an ‘outer removal area’ (‘buffer area’ 210 km2) and a ‘control area’ (1456 km2) of the EOP (collectively, an area of 2194 km2 in the Irish midlands), and a ‘neighbouring area’ (966 km2; covering all areas of County Offaly not previously included in the EOP). These areas are located in the Irish midlands, which is comprised of a mixture of productive pasture land, low-lying area and bog. The areas shared similar characteristics in terms of cattle husbandry, land type and land use [Reference Ó2, Reference Eves3]. Further, rates of bovine TB in the study areas were similar immediately prior to the start of the observation period; in 1988, the rate of reactor animals per thousand animal tests was 3·9 and 3·4, in the removal and control areas, respectively [3, table 5] and 3·1 in the neighbouring area. The removal and control areas are also described elsewhere [Reference Ó2–Reference Ó4]. Briefly, herds in the inner and outer removal areas were located at least, and less than, 1 mile (1·6 km) inside the boundary of the removal area, respectively. Herds in the control area were located with defined district electoral divisions (DEDs). The removal areas were located entirely within County Offaly (apart from 50 herds in County Laois and one in County Kildare), whereas the control area spanned areas in counties Kildare, Laois, Meath, Offaly and Westmeath. The study areas were arranged in the shape of a doughnut (Fig. 1). In both the EOP and the current investigation, fragmented farms (i.e. those with more than one land fragment) were allocated to a study area according to the location of the home farm (the fragment containing either the farmyard or the fragment where most cattle were located). We identified a subset of herds in the control area, (those <2 km from the removal area boundary, total area about 289 km2) based on the location of the centroid of the land parcel containing the home farm.
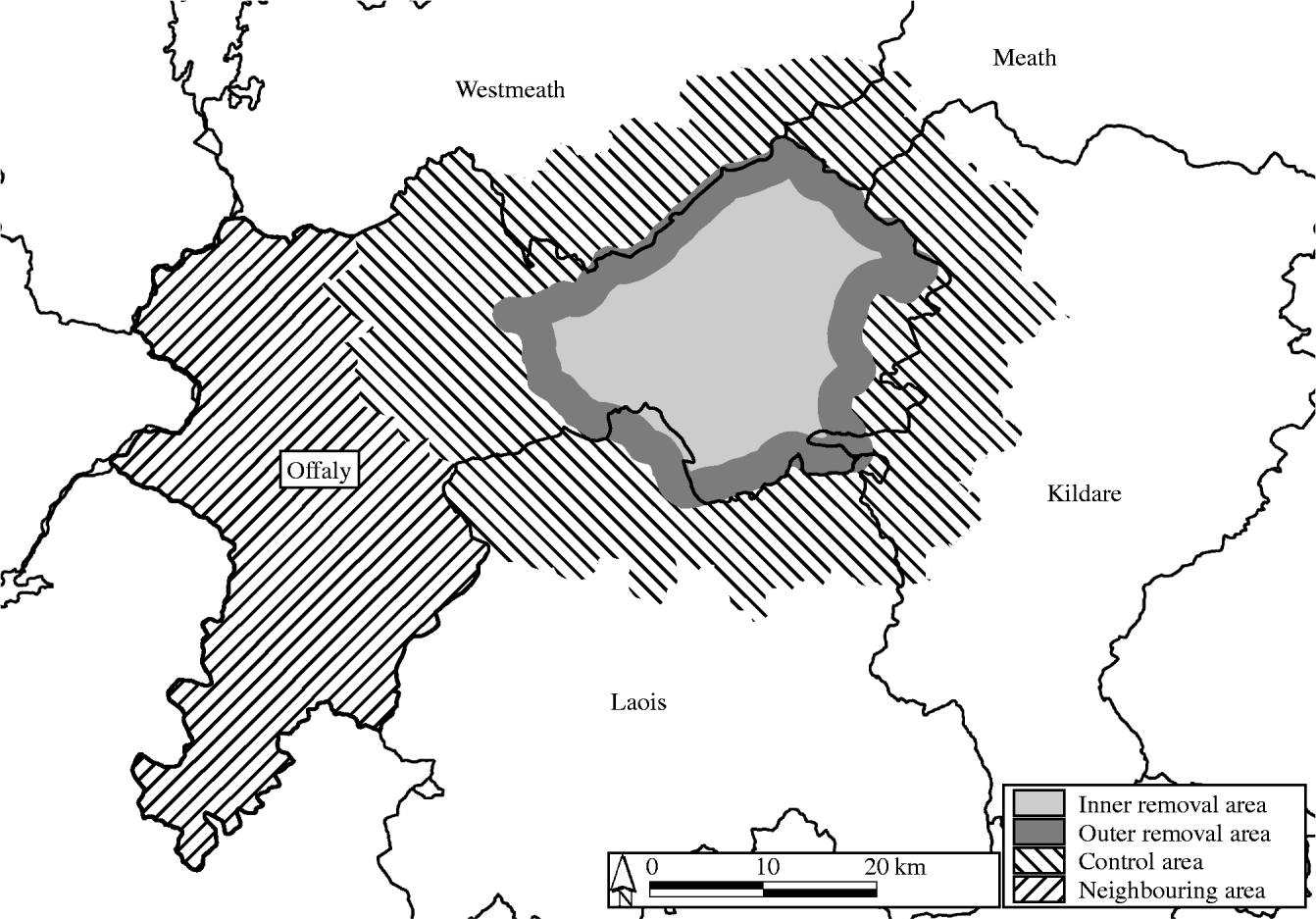
Fig. 1. Location of the investigated areas in the Irish midlands.
In Ireland, digitized data are available of each of the land fragments on all farms that applied for EU area aid between 1999 and 2004. This included 58% of herds in the removal and control areas. These data are managed by the national Department of Agriculture, Fisheries and Food, within the Land Parcel Information System (LPIS). We used these data to calculate the distribution of land fragments for individual herds within a number of defined geographic regions.
The investigation period
The investigation was conducted to cover the years 1989–2004, including 7 and 9 years during and following the EOP, respectively.
Badgers
General information about the density of badgers in the Irish midlands was obtained from the National Badger and Habitat Survey [Reference Smal17], conducted between 1989 and 1993.
The removal areas
Commencing in January 1989 and for 7 years to December 1995 (i.e. the formal study period of the EOP), proactive badger removal was conducted twice each year in spring and autumn in the inner and outer removal areas [Reference Eves3]. From 1996, proactive badger removal continued once each year in spring and autumn, except in the spring of 1997, 2001 and 2003 (Table 1). Badger removal was authorized under license from Dúchas, the National Wildlife Service.
Table 1. The number of herds at risk, the number of herd restrictions and % restrictions due to bovine tuberculosis in four areas of the Irish midlands during 1989–2004

The control and neighbouring areas
In these areas, badger removal was conducted reactively in accordance with national policy. In response to a severe confirmed TB outbreak (an outbreak where two or more standard tuberculin reactors were disclosed and one or more lesions) in either a herd or cluster of herds, a Veterinary Inspector (VI) could apply for a licence for badger removal (for up to a 2 km radius from the index farm), provided badger involvement was implicated on the basis of a detailed epidemiological investigation. Setts were recaptured until there was no further activity. This national policy was applied consistently in County Offaly (covering the neighbouring area and part of the control area) throughout the full investigation period. Although this policy was equally applicable to other counties in the Irish midlands during this period, implementation was conducted separately by the District Veterinary Office in each county. Therefore, there may have been some county-level differences in the application of this policy within the control area.
Cattle
In common with the national tuberculin testing programme, all herds in the areas investigated were tested for TB on an annual basis throughout the period of interest. A herd was restricted, and could not trade or move cattle, except under permit for slaughter, if infection was detected at this annual test, or during routine abattoir surveillance. The restriction was confirmed if a tuberculous lesion was disclosed in one or more reactor animals at post-mortem examination. A restricted herd was retested every 60 days after removal of reactors, until two clear consecutive tests were obtained, when the restriction was lifted. (Further details are described in refs [Reference Ó2, Reference Ó4].)
Statistical methods
Herd-level data between 1989 and 2004 were examined using survival analysis. Our outcome of interest was the time to a confirmed restriction or time under observation. Each herd was enrolled from 1 January 1989, the date when a TB restriction was first lifted following a restriction spanning this date, or the date when a herd number was initially assigned, whichever occurred last. Each herd could experience more than one confirmed restriction over time (leading to multiple survival times, starting from the date when the previous confirmed restriction was lifted); in subsequent modelling, repeat restrictions were indicated using a variable describing the number of previous restrictions (at investigation start, initially assigned to zero). If herds were free to trade on 31 December 2004, then data were right-censored on this date. If a herd became inactive (no herd test recorded for more than 2 years), then data were right-censored from the date of the last herd test.
A Cox model was fitted to these data and rates of TB in all areas were compared with the control area forming the baseline group. The start-stop form (counting process form) of the Cox model was used, with the Anderson–Gill method for treating multiple events [Reference Therneau and Grambsch18]. This allowed the use of a time-varying area effect, including a time-varying effect of badger removal, and the calculation of model diagnostics. Each herd was represented as one or more observations, each consisting of a time interval, the censoring status and fixed covariate values over that interval. Herd size (calculated as the natural logarithm of the median of the last three full herd tests) and number of previous restrictions were each considered time-dependent covariates in the models. The area effects – removal and neighbouring, were modelled as time-dependent covariates; nonlinear time effects using spline methods and polynomial effects in time were considered. The interval unit of time for the time-varying area effects was chosen as 1 year. Choosing shorter intervals of time did not change the results appreciably but increased computational time by a large factor. Since repeated survival episodes in the same herd could not be assumed to be statistically independent, a jackknife method (in addition to the variable describing the number of previous restrictions) was used to adjust the variances of parameter estimates from the models to account for correlation between repeated observations [Reference Collett19]. Interaction terms involving areas, herd size and previous history were also considered for inclusion in the model. A series of forward and backward elimination steps were performed using the adjusted standard errors to select the variables upon which the hazard function depended.
RESULTS
The investigation areas and herds
Table 1 presents the number of herds at risk, and the percentage restricted, in the four investigation areas during 1989–2004. During this period, 67% of restrictions were confirmed. The normal testing protocol was interrupted only briefly in 2001 as a result of the foot-and-mouth disease epidemic.
Of the farms allocated to the inner removal area, 84% of their land area was located in this area. A further 10% of their land area was located in the outer removal area, 4·6% in the control area and 1·4% elsewhere. In total, the land area was entirely contained within the inner removal area for 71% of herds so allocated. Of the farms allocated to the control area, 88% of their land area was located in this area. A further 2% of their land area was located in the inner removal area, 3% in the outer removal area and 7% elsewhere. In total, the land area was entirely contained within the control area for 70% of herds so allocated.
(x,y)-coordinates were available for 2517 of the 3442 herds (73%) in the control area over the course of the study. The percentage in the neighbouring area was 74% and in the removal areas 80%. Coordinates were unavailable mostly for small herds entering in the study pre-1997 before the digitization of farms as described above, and that subsequently ceased to trade. By 1997, the number of herds without recorded (x,y)-coordinates in the control area had reduced to 283. We note the mean herd size for control herds with recorded (x,y)-coordinates ⩽2 km and >2 km from removal area boundaries was 64 and 62 cattle, respectively, while for control herds without (x,y)-coordinates it was 22. The restriction rates for herds in the control area with recorded (x,y)-coordinates were higher throughout the course of the study than those without: 5·61% and 1·41%, respectively, by 2004. Control herds that could be identified as ⩽2 km from culling area boundaries were designated as ‘inner’ and the remainder as ‘outer’ control herds. Thus some herds, mostly smaller ones, were misclassified as ‘outer’.
Badgers
The Irish midlands were identified as an area of high estimated badger density [Reference Smal17]. The density of badgers in all studied areas was similar.
Table 2 presents the number of badgers removed annually, the percentage infected with Mycobacterium bovis and the annual average removal intensity (badgers removed per km2 per year) between 1989 and 2004, from the four areas. In the inner and outer removal areas, about 29 000 individual sett visits were conducted during 24 separate removal operations during 1989–1994, and the percentage of active setts (i.e. setts with signs of badger occupation) declined from 70% in 1989 to 9% in spring 1994 [Reference Eves3]. In the inner removal area, the average annual removal intensity was 0·34 and 0·14, and in the outer removal area 0·36 and 0·18, during 1989–1995 and 1996–2004, respectively. In the control area, the average annual removal intensity during these periods was 0·01 and 0·04, and in the neighbouring area 0·12 and 0·11, respectively. In the inner removal area, the percentage of infected culled badgers was 12% and 6% during 1989–1995 and 1996–2004, respectively, and in the outer removal area the corresponding figures were 8% and 11%. In the control area, the percentage of infected culled badgers was 4% during 1996–2004, and in the neighbouring area 10% and 13%, during 1989–1995 and 1996–2004, respectively. The difference between the two time periods was significant only in the inner removal area (a reduction of 6%, 95% CI 5·8–6·6, Fisher's exact test P<0·001).
Table 2. The number (and % infected) badgers removed, number and percentage of infected badgers removed, and removal intensity, in four areas of the Irish midlands by season and year during 1989–2004
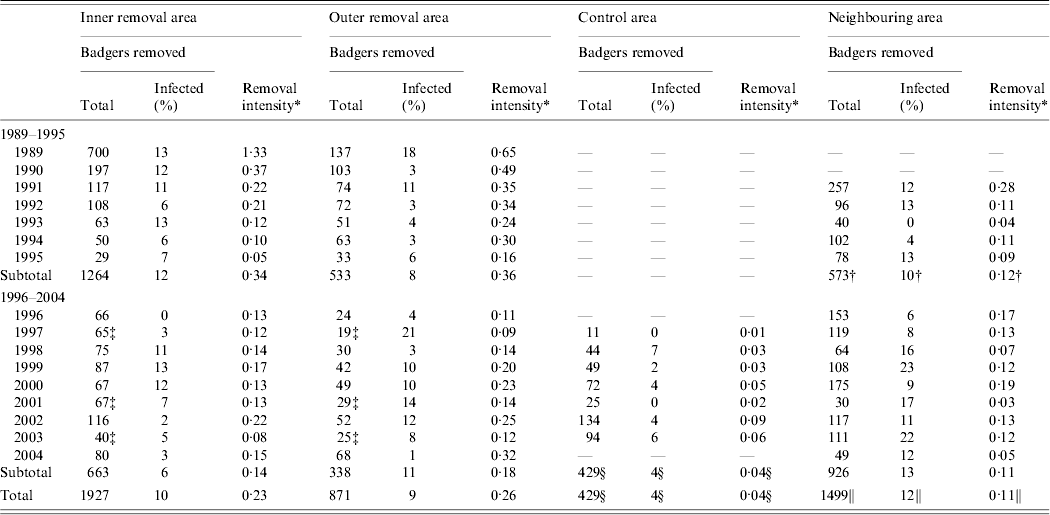
* Number of badgers removed per km2 per year.
† Five years (1991–1995) only.
‡ Single capture (autumn) only.
§ Seven years (1997–2003) only.
ǁ Fourteen years (1991–2004) only.
— , Data not available.
Statistical modelling
The final model had four significant covariates: area (inner and outer removal areas and neighbouring area), log herd size, number of previous restrictions, and the two-way interaction between herd size and presence or absence of previous restrictions. The estimated coefficients for this model are shown in Table 3, along with unadjusted and robust (i.e. jackknife adjusted) standard errors, P values and hazard ratios. The treatment effect for the inner removal area varied over time. Polynomial terms as well as spline methods were used to model this temporal effect and it was found to be best modelled with a nonlinear treatment(inner)×log(time) interaction term (P=0·01). Figure 2 a shows a plot of the hazard ratio for the inner removal area over the control area as a function of time. This shows a steep decrease in the first few years of the investigation, followed by a period of a more gradual decrease to the end of 2004. The hazard ratio was significantly <1 by early 1990 (hazard ratio 0·87, 95% CI 0·75–0·99, P=0·040), had decreased to 0·72 (95% CI 0·65–0·81, P<0·001) by the end of 1994 and to 0·63 (95% CI 0·53–0·75, P<0·001) by the end of 2004. In addition, there was a lower hazard of a confirmed restriction in the inner compared to the outer removal area from mid-1995 (Fig. 2 b); the hazard ratio for inner over outer removal area was 1·00 (95% CI 0·81–1·24, P=0·98) in 1990, became significantly <1 (0·82, 95% CI 0·68–0·99, P=0·04) by mid-1995, and decreased steadily to a value of 0·72 (95% CI 0·57–0·92, P=0·007) by the end of the investigation period. Herds in the outer removal area had a lower hazard of a confirmed restriction compared to the control area (0·87, 95% CI 0·73–1·03, P=0·11), but the effect was constant over time (Fig. 2 c). When the inner and outer removal areas were combined, the overall hazard was lower than the control area (0·78, 95% CI 0·71–0·85, P<0·001) and constant over time [the treatment×log(time) term was found to be only of borderline significance, P=0·055]. The hazard ratio between the neighbouring and control areas varied over time [Table 3, Fig. 2 d, positive intercept, negative slope over log(time)] but was not significantly different from 1 except for the first year of investigation when the neighbouring area is slightly higher. The hazard ratio was 1·19 in 1989 (P=0·028), had decreased to 0·99 (P=0·881) by 1995, and was 0·92 by the end of 2004 (P=0·160).
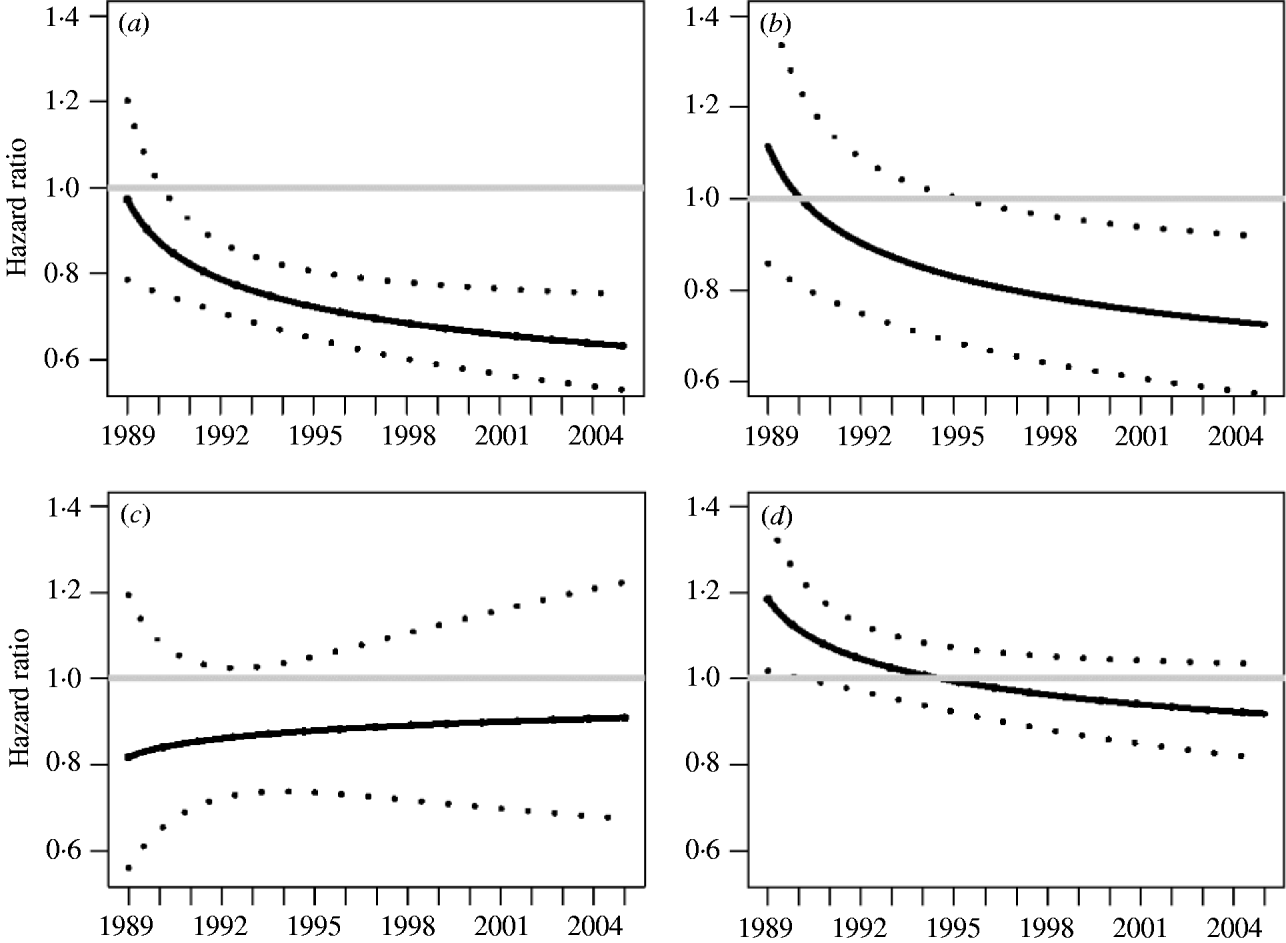
Fig. 2. Hazard ratio (with 95% confidence limits) of a confirmed herd restriction during 1989–2004 for: (a) the inner removal area over the control area; (b) the inner removal area over the outer removal area; (c) the outer removal area over the control area; (d) the neighbouring area over the control area.
Table 3. Proportional hazards regression estimates of the hazard of a confirmed herd restriction due to bovine tuberculosis with the control area as baseline, in the Irish midlands during 1989 to end of 2004
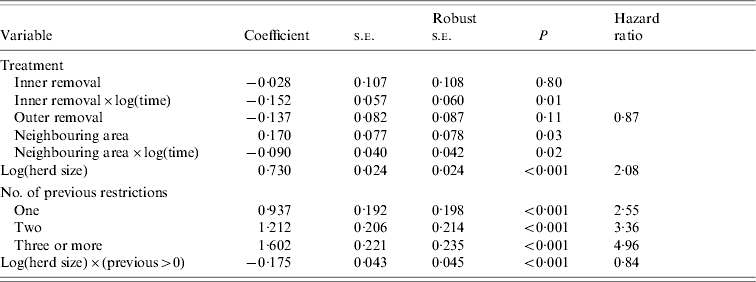
s.e., Standard error.
The hazard ratio of the inner removal area over the neighbouring area was <1 in 1989 (hazard ratio 0·82, P=0·074), was 0·73 by the end of 1994 (P<0·001) and 0·69 (P<0·001) by the end of 2004. The hazard ratio of the outer removal area over the neighbouring area was significantly <1 in 1989 (hazard ratio 0·74, P=0·005), was 0·88 by the end of 1994 (P=0·100) and 0·95 (P=0·616) by the end of 2004. The hazard of the entire removal area over the neighbouring area was 0·77 (95% CI 0·70–0·84, P<0·001) by the end of 2004.
In a model restricted to control herds with (x,y)-coordinates, herds in the inner control area had a lower hazard than those in the outer (0·83, 95% CI 0·73–0·93, P=0·002), and the effect was constant over time. In addition, the model in Table 3 was re-fitted with inner and outer control herds (all) considered as separate groups. The inner control had a lower hazard than the outer control (0·86, 95% CI 0·76–0·97, P=0·013) and was constant over time. The outer removal area was not significantly different from the inner control (0·99, 95% CI 0·81–1·20, P=0·890) in this model. The hazard of the neighbouring area was higher than the inner control for all years, going from 1·34 in 1989 (P=0·001) to 1·04 in 2004 (P=0·656). It was significantly higher from 1989 to 1994. The hazard of the neighbouring area over the outer control was 1·15 (P=0·069) in 1989, it had decreased to 1·00 (P=0·882) by 1993, and further decreased to 0·89 (P=0·057) by 2004. In a model restricted to outer control and neighbouring herds with (x,y)-coordinates, the hazard ratio of the neighbouring area over outer control went from 1·05 in 1989 to 0·87 in 2004 (P=0·044). However, the time-varying effect was not significant (P=0·181) and the overall hazard ratio was 0·94 (95% CI 0·87–1·01, P=0·100).
An interaction between herd size and number of previous restrictions was significant (P=0·01) and was adequately described by an interaction between herd size and presence or absence of previous restrictions. The other two- and three-way interactions between herd size, areas and number of previous restrictions were not significant. There was an increasing hazard for a second or subsequent restriction. Herds with one previous restriction had a 2·5-fold increase (95% CI 1·7–4·0, P<0·001) in the risk, herds with two previous restrictions had a 3·4-fold increase (2·2–5·1, P<0·001) in risk and herds with more than two previous restrictions had a 5·0-fold increase (3·1–7·9, P<0·001) in risk of a subsequent restriction compared to herds with no restriction. For all herds, the effect of log(herd size) was significant (P<0·001) with a doubling of herd size corresponding to a 1·66-fold increase in risk for those herds with no previous history and a 1·47-fold increase for herds with a previous history. However, this reduction in hazard for larger herds at risk of a second or subsequent restriction was moderated by the increased hazard associated with having previous restrictions. For the majority of herds represented in these data, the hazard of a second or subsequent restriction was higher than that of a first restriction.
Plots of martingale residuals and dfbetas (influence) residuals showed no evidence of model inadequacy.
DISCUSSION
This investigation was undertaken to gain a clearer understanding of the long-term effects of both proactive and reactive badger removal on confirmed herd breakdowns. The current investigation provides unique insights into these questions, given the availability of data both during and subsequent to the formal 7-year project, as well as measured changes in the intensity of proactive badger removal over time. However, the study was observational, and relevant events did change over time. For example, during the first 7 years of the observation period (1989–1995), there was an intensive, coordinated and well-resourced programme of proactive badger removal, overseen (in all but 1995) by a single project veterinarian. Subsequently, and as a consequence of changes in policy, resources and personnel, the programme continued, albeit with substantially reduced coordination and intensity. As highlighted in Table 2, these changes are reflected both in terms of the pattern and number of badger removals. For these reasons, the results of observational studies, such as this, should be interpreted with care.
Proactive badger removal was associated with a significantly reduced risk of TB breakdowns in associated cattle herds (Fig. 2 a, Table 3). This result is in agreement with earlier reports from both Ireland [Reference Eves3, Reference Griffin5, Reference Griffin6] and Britain [Reference Donnelly9, Reference Donnelly12], and is consistent with our understanding of the contribution of badgers in the epidemiology of bovine TB in Ireland. The current investigation also provides additional insights. Proactive badger removal was more effective than reactive badger removal in reducing TB breakdown risk, with an overall reduction in incidence of confirmed herd restrictions in the removal area of 22% compared to the control area (Fig. 2 a, c, Table 3). The effect was less marked in areas of ongoing badger immigration (Fig. 2 b, Table 3, reduction in incidence 13%, outer removal area vs. control area) which agrees with earlier RBCT findings [Reference Donnelly12] of an increasing effect of culling with increasing distance inside the culling area boundary. Further, there was a rapid decline in breakdown risk in the inner removal area (compared to the control area) following the implementation of the intensive (four times yearly) programme of proactive badger removal. This effect was sustained, indeed it continued to fall, over a total of 16 years, encompassing periods of intensive and less-intensive badger removal. Similar temporal efforts were observed in the RBCT [Reference Donnelly12], where a trend was observed of increasing positive effects of proactive culling over time. Table 2 indicates quite differing levels of badger capture over the years in the removal areas, perhaps reflecting the addition to changes in policy and personnel noted earlier, and depletion of the badger population over time. The RBCT also reported that more thorough badger removal was sustained on later culls [Reference Woodroffe20]. We note the effects seen here were also seen when comparing the removal to the neighbouring area. There was a decrease in the incidence of confirmed herd restrictions, sustained over 16 years, in the inner removal area compared to the neighbouring area, attaining a value of 31% by 2004 (P<0·001). The decrease for the entire removal area over the neighbouring area was 23% by 2004 (P=0·001). In this investigation, we have assessed the effect of proactive culling in comparison with the control area (and also the neighbouring area), where localized reactive culling was conducted. In the absence of a no-cull control, there is the potential to overestimate the true effectiveness of proactive culling [Reference Donnelly7], specifically if reactive culling is associated with an increased TB incidence in comparison with a no-cull control. However, there was no evidence of this effect in the FAP [Reference Griffin5, Reference Griffin6]. In the current investigation, we also have no evidence of effect overestimation, as discussed later.
In recent years, the effect of proactive badger culling has now been observed in three separate studies, including the EOP [2–4; updated in the current investigation] and FAP [Reference Griffin5, Reference Griffin6] projects in Ireland, and the RBCT [Reference Donnelly9, Reference Bourne11, Reference Donnelly12] in Britain. In each of these studies, cattle TB incidence was reduced following badger removal, providing irrefutable evidence of the role of badgers in the epidemiology of bovine TB. Nonetheless, the magnitude of this effect was different in each study. In the current investigation, the adjusted hazard ratio for the inner removal area at the end of 2004 was 0·63, equivalent to a reduction in the incidence of confirmed herd restrictions of 37% (95% CI 25–47, P<0·001). The reduction was 22% for the entire of the removal area (95% CI 14–29, P<0·001). The adjusted hazard ratios in the FAP study areas (inner removal areas vs. reference) ranged from 0·28 to 0·52 [5, table 8], equivalent to a reduction of 48% (31–61%) to 72% (47–85%). In the RBCT, the overall figure for removal areas was 23% (12·4–32·7) [Reference Donnelly12]. There are a number of potential explanations for these observed differences in effect. Prior to the beginning of observation in each of the areas investigated, badger density was likely to have been considerably lower in Ireland than Britain [Reference Griffin5]. Further, the effectiveness of the proactive removal operations (affecting residual badger density following removal operations) were probably higher in Ireland than in Britain, due to factors affecting compliance and illegal interference, the method of culling, the frequency and duration of culling, and (in the case of the FAP) the relative permeability of the study area boundaries [Reference Griffin5, Reference Bourne11]. However, badger removal was more thorough deeper inside trial areas in the RBCT [Reference Woodroffe20], perhaps contributing to reductions in these areas comparable to those seen in the FAP. Herd selection criteria were also different between the FAP on the one hand, and both the RBCT and the current investigation on the other. In the FAP, the area comparison included only those herds wholly contained within each removal or reference area [Reference Griffin5]. In this investigation, however, farms were included based on the location of the farmyard, resulting in an expected dilution in any treatment effect (herds assigned to the removal areas with fragments in the control area, and vice versa). The latter approach was also used in the RBCT [Reference Donnelly9, Reference Bourne11, Reference Donnelly12].
This investigation provides some insights into the long-term effects of reactive culling on TB prevalence in cattle. There was no evidence of adverse effects of proactive culling in areas immediately adjoining proactive trial areas. The rates of confirmed herd restrictions in the outer removal area and inner control area were not significantly different (P=0·890). In contrast in the RBCT, overall cattle TB incidence was 24·5% higher on land up to 2 km outside proactive trial areas [Reference Donnelly12], compared to no-cull areas, although the effect was of borderline significance (P=0·057). We note that in this study, unauthorized badger culling may have occurred in the control area, following the publication of the EOP study [Reference Eves3]. Further, there was a significant reduction in confirmed herd restrictions in the inner compared to the outer control areas of either 17% (P=0·002, geo-referenced herds only) or 14% (P=0·013, all herds). The latter figure is smaller, due perhaps to the misclassification of some smaller herds, which generally have a lower hazard for TB than large herds. One explanation for the difference in inner and outer control areas, is that badgers may have moved from the control into the removal areas (as reported for the earlier years of this study [Reference Eves3]), affecting the inner control area to a greater extent than the outer control area. Thus, beneficial effects of culling observed in the removal areas may have extended into the inner control area. These results comparing the outer removal, inner control and outer control areas, seem to indicate that no adverse effects follow reactive badger culling. If reactive badger culling led to increased cattle TB it is most likely to be observed at the interface between proactive and reactive badger removal. This was not observed in the inner control area where badger disturbance and associated movement is possibly at its highest. A range of reasons for differences between Irish and British results have been proposed [Reference Griffin5, Reference Bourne11], including factors relating to badger abundance, removal intensity, permeability of study area boundaries and cattle management. There was also a sustained fall in confirmed herd restrictions in the control and neighbouring areas between 1989 and about 1998, as in the removal areas, and the rate subsequently rose as it did in the outer removal area (and to a lesser extent), in the inner removal area. However, in the absence of a no-cull control, these trends could be unrelated to culling and simply be culling-unrelated temporal trends.
We compared the control and neighbouring areas. There were no statistically significant differences between the control and neighbouring area over the study period (Table 3, Fig. 2 d). We note however, that the rate for the neighbouring area was higher until 1993 and lower thereafter, with a comparative reduction of 8% in confirmed herd restrictions by 2004 (P=0·160). Examining this effect in more detail, the inner control area had a reduced confirmed herd restriction rate compared to the neighbouring area for all years, going from 34% in 1989 to 4% in 2004 (conservative estimates due to misclassification, particularly in earlier years). It was significantly reduced from 1989 to 1994 when perhaps badger movement into proactive areas was highest. The outer control area had a lower confirmed herd restriction rate than the neighbouring area of 15% in 1989, the two areas had equal rates by 1993, and the neighbouring area was lower by 11% (P=0·057, all herds) or 12% (P=0·044, geo-referenced herds) by 2004 (however, the time-varying effect for the latter was not significant and the overall constant reduction was 6%, P=0·100). These differences may be due to differing levels of badger removal intensity (Table 2) with a considerably lower rate in the control than neighbouring area, the latter appearing to have remained relatively stable over the years. The difference may also be due to badgers' daily ranges increasing and increased dispersal in the control area due to proximity to proactive areas, as in the RBCT [Reference Woodroffe20] and EOP [Reference O'Corry-Crowe21]. These two possible effects for the differences are confounded, but either may have led to higher rates of contact of badgers with cattle in the outer control area compared to the inner or neighbouring areas, resulting in higher (not significant) cattle TB rates in the former by 2004. Overall by 2004, there is little difference between the inner control, outer removal and neighbouring areas in terms of confirmed herd TB restrictions, while the outer control has a somewhat higher restriction rate and lower badger removal intensity.
These results for the control compared to the neighbouring area are somewhat at variance with the results from the RBCT, as noted above. Unlike the RBCT, the positive effect of proactive culling extended into the inner control area in this study, although this effect diminished with time. However, recent concluding analyses from the RBCT [Reference Donnelly12], indicate that the differences in findings with the Irish studies may not exist in the long term. In areas ⩽2 km outside proactive culled areas in the RBCT, the detrimental effect of culling diminished over time [Reference Donnelly12].
There was a decrease in M. bovis prevalence in badgers due to proactive culling (Table 2). This is similar to the findings of the FAP [Reference Griffin5] but different to the RBCT [Reference Woodroffe22], where prevalence rose markedly on successive culls. The difference was noted [Reference Bourne11], and was attributed to ecological differences between the RBCT and Irish study areas, namely permeability of RBCT boundaries and low background badger density in the Irish areas.
There was no significant difference in M. bovis prevalence in badgers in the neighbouring area between 1989–1995 and 1996–2004 and thus we found no evidence to indicate that reactive culling leads to an increase in M. bovis prevalence in badgers.
In common with results from the FAP [Reference Griffin5, Reference Griffin6], past history, herd location and herd size were each important predictors of future breakdowns. In the current investigation, about 33% of herds with a previous restriction experienced at least one further restriction during the observation period. Herd location is considered a key risk factor for TB in Ireland, as highlighted by the stable pattern of spatial clustering throughout the country [Reference More1]. Knowledge is incomplete about reasons for persistence of infection in defined ‘hotspot’ areas in Ireland, and not elsewhere. It is likely that residual infection in both wildlife and cattle are each important. Infection in badgers persists locally, since these animals tend to re-colonize the same setts [Reference Cheeseman23]. Data will soon be available on the geographic variation in infection prevalence in Irish badgers. Larger herds were at increased risk of a confirmed restriction over smaller herds [Reference Ó2, Reference Griffin5]; among herds with no previous restriction, there was a 1·7 increase in risk as herd size doubled. In common with earlier findings [Reference Ó2], this increase in risk was reduced for herds with previous restrictions. We also note there was a 30% decrease in the number of herds at risk as time progressed. This is due to a trend towards larger farms, which is a national phenomenon.
Issues associated with the use of certain models of dependency in multiple events have been previously discussed [Reference Therneau and Grambsch18]. All the models assume multiple survival times for a herd are independent and any possible correlation is adjusted for using a robust (jackknife) estimate of variance. An alternative approach is to model the dependency with a frailty term. This was done for a subset of these data by Kelly & Condon [Reference Kelly, Condon, Hinde, Einbeck and Newell24] using a gamma distribution for the frailty and the results of the fit were similar to those here. An attempt was also made to fit a non-parametric frailty distribution [Reference Kelly, Condon, Hinde, Einbeck and Newell24], but the algorithm did not converge. Such a distribution might, for example, indicate a possible categorization of herds, e.g. ‘good’ and ‘bad’. The models discussed in Kelly & Condon [Reference Kelly, Condon, Hinde, Einbeck and Newell24] differ in the time-scale chosen for the baseline hazard. The Anderson–Gill model was considered as the most appropriate here as it uses calendar time and thus, for example, if environmental factors affect TB rates then they are incorporated in the baseline hazard and are common to all herds at risk at the same calendar time, regardless of previous history. The previous history effect is added to the model as a covariate. However, the number of previous restrictions is subject to the effect of the two culling treatments and may confound the interpretation of the treatment effects when modelled in this way. We note the interactions treatment×previous, and herd size×treatment×previous were not significant in our models so this was not a problem. Moreover, as noted in Clifton-Hadley et al. [Reference Clifton-Hadley25], these data pose difficulties for analysis since herds in the same area cannot be considered to be truly independent. Work is underway on spatial models for these data and the FAP to account for possible correlation between herds.
In conclusion, this investigation provides further evidence of the importance of badgers in the epidemiology of bovine TB in Ireland. Proactive badger culling was associated with a significant and sustained decrease in disease risk in associated cattle herds, relative to reactive culling. Overall, the risk decreased with time. The effect of proactive culling was greater in the inner as opposed to the outer removal area. In this study, there was no evidence of increased disease risk in cattle at the interface between areas of proactive and reactive badger removal. These findings should contribute to policy decisions during this interim period, pending the development of an effective TB vaccine for badgers.
ACKNOWLEDGEMENTS
We thank Daniel Collins for assistance with mapping, Guy McGrath and Dr R. Hammond for geo-references (all of CVERA) and two anonymous referees for helpful comments.
DECLARATION OF INTEREST
None.







