INTRODUCTION
Documentation of the incidence and morbidity of paediatric influenza and respiratory syncytial virus (RSV) infections informs immunization policy. In the United States, such epidemiological data have been used to great effect in bringing about recommendations for universal paediatric influenza immunization and will be used to create RSV immunization recommendations when an ideal vaccine becomes available [Reference Izurieta1–5]. In continental Europe there are limited paediatric influenza data available for Germany, [Reference Weigl, Puppe and Schmitt6] Spain [Reference Navarro-Mari7] and France [Reference Ploin8] but there is no consensus on universal immunization. In the United Kingdom, a recent study involving children <72 months of age in a large hospital in Leicestershire [Reference Nicholson9] revealed that 5·4% of children presenting to the hospital with acute respiratory illness had a diagnosis of influenza infection.
RSV remains the commonest cause of acute lower respiratory tract infections in infants in the industrial world. It is responsible for hospitalization rates of about 30–40/1000 infants per year in Europe and North America, and 59/1000 infants per year in Japan in those with no risk factors [Reference Muller-Pebody10–Reference Saijo14]. Few population-based RSV epidemiological studies exist in Europe and the impact has largely been demonstrated in the context of severe disease and hospitalization rates, particularly with regards to high-risk groups [Reference Weigl, Puppe and Schmidt15, Reference Eriksson16].
Furthermore, the interpretation of influenza and RSV incidence rates and morbidity data is often constrained by the knowledge that there may have been a degree of under-reporting. In order to obtain outpatient visit and hospitalization rates attributable to paediatric influenza, differences from the baseline rates in a Massachusetts health maintenance organization were calculated over five seasonal study periods in a retrospective study [Reference O'Brien4]. The rates of outpatient visits attributable to influenza and RSV among infants and children aged 6–23 months were 1·8 and 4·6/100 person-months, respectively, while for hospitalization this excess rate was 3·9 and 8/10 000 person-months, respectively.
There still remain differences in opinion within European countries about universal influenza vaccination for children [Reference Principi and Esposito17]. Paediatric influenza and RSV incidence and morbidity data gathered at the local or regional level may help to inform vaccination policy not only at those levels but also at the national level provided it is considered to be representative. We gathered hospital data prospectively during two seasons 2002–2003 and 2003–2004 from a large paediatric population in the catchment area of the Royal London Hospital (RLH), Tower Hamlets, East London in the United Kingdom, a country where universal paediatric influenza immunization is not yet recommended.
METHODOLOGY
Study design
This study was conducted at the RLH in East London. It was a prospective, descriptive study of infants and young children aged <6 years presenting with symptoms suggesting influenza. The study was performed over two winter seasons from 14 October 2002 for 31 weeks and from 17 November 2003 for 24 weeks. The local research ethics committee of the East London and City Health Authority gave ethical approval.
Study population
The main catchment area of the RLH is the London borough of Tower Hamlets, which accounts for about 17 889 resident infants and children aged <6 years [18]. Of those routinely seen at RLH Accident and Emergency Department (A&E), about 80% reside in Tower Hamlets. By contrast, a small proportion of infants and children (1·3%) that were resident in Tower Hamlets were treated routinely at the A&E of the Homerton and Newham Hospitals during the study periods. These are general hospitals on the fringes of the borough of Tower Hamlets. All the children were recruited from six paediatric hospital wards, the high dependency unit or the paediatric A&E of the RLH.
Inclusion and exclusion criteria
Any infant or child aged <6 years was eligible for recruitment if presenting to the hospital with a history of one or more of the following conditions [which collectively define ‘influenza-like illnesses’ (ILI) in children for the purpose of this study] for <7 days (168 hours).
Inclusion criteria were:
• acute upper respiratory tract infections including colds, sinusitis, pharyngitis (red sore throat), tonsillitis (inflamed tonsils) and acute otitis media;
• acute lower respiratory tract illnesses including croup, tracheitis, bronchiolitis (wheeze age <12 months), virally induced wheeze, asthma and pneumonia (asthma was defined as a child aged >2 years with a history of asthma or newly diagnosed during the study);
• any seizure;
• any acute febrile gastroenteritis without rebound tenderness or guarding;
• apnoea or any life-threatening event in infants aged <12 months;
• any acute febrile illness (temperature ⩾38·0°C) but excluding any condition with an acute rash or known non-respiratory viral illness.
Exclusion criteria included any contraindication for nasopharyngeal aspiration (NPA), i.e. recent nasopharyngeal surgery or acute epiglottitis. Those presenting to A&E outside study recruiting times (08:00–18:00 hours) were not recruited overnight but if admitted to hospital, they were eligible for recruitment the next day. Those with difficult social problems such as child protection issues were not approached. In order to obtain written informed consent parents or guardians had to be fluent and literate in English or Bengali (Bengali interpreters were used). Patients were also excluded if consent was refused.
Study team
One clinician and two research nurses were employed during the two seasons. The hospital-based recruitment took place between Monday and Friday in both seasons and also on Saturday in the first season.
Patient enrolment and evaluation
Medical staff in the A&E and the paediatric wards notified the research team about eligible participants. These staff undertook diagnosis and management, according to RLH standard clinical protocols. Once informed consent was obtained enrolment required:
(1) Completion of an electronic standardized questionnaire recording patient demography, past medical history, clinical findings, investigations and diagnosis details. Details of pre-hospital and hospital management were also collected.
(2) A NPA was performed within 24 h according to standard procedures and samples were sent for viral immunofluorescence (IF) and polymerase chain reaction (PCR). The latter was performed twice on every sample; molecular testing of samples was performed using the assays described for influenza by Poddar et al. [Reference Poddar, Espina and Schnurr19], for RSV by Osiowy [Reference Osiowy20], for enterovirus by Shen et al. [Reference Shen, Desselberger and McKee21] and for adenovirus by Echavarria et al. [Reference Echavarria22]
(3) Telephone follow-up with parents took place at 7 (±2) days, 14 (±2) days and 21 (±2) days following discharge home and weekly until there was objective evidence of resolution of the clinical syndrome. A structured questionnaire was administered during these follow-ups. During telephone contacts, test results were relayed and information was collected on:
• the child's clinical state;
• whether or not they required re-attendance at a health-care facility;
• whether or not additional medications had been procured;
• school or day-care absenteeism;
• parental time taken off work;
• other direct societal costs of treatment.
An anonymized database was set up for all eligible children that were not recruited so that baseline comparisons and extrapolations could be made between the recruited and the unrecruited. This database recorded the date the child was seen, the age, sex, diagnoses and whether admitted to hospital.
Virological studies
Indirect IF methods were used to detect RSV, influenza viruses A & B, parainfluenza virus and adenovirus on fresh NPA aspirates. Separate samples were collected in buffer-containing Eppendorf bottles and stored. A multiplex PCR technique was performed on stored samples to detect RSV, influenza virus A & B, parainfluenza 1, 2 and 3 viruses and enterovirus RNA. PCR analysis was performed twice on each NPA sample in the two seasons.
Statistical analysis
All statistical tests were carried out in stata 8.0 [23]. Incidence rates were calculated for the population of Tower Hamlets as the denominator population was reasonably accurately described. As virology was not available for non-recruited cases, their virology infection rates were estimated from knowledge of the in-patient/outpatient status, clinical diagnosis, and age, using corresponding data of virology status in those recruited. Numerators were corrected to reflect the proportions of eligible cases presenting to A&E and residing in Tower Hamlets (i.e. 80% of all attendees resided in the borough of Tower Hamlets). Numerators were also corrected to reflect the fact that 86·5% and 81·5% of children with influenza and RSV-positive virology were from the borough, respectively. Further extrapolations were made as some children had more than one diagnosis and this was done by adjusting the estimated number of cases based on the actual number of observed cases in each age stratum. Age-specific incidence rates were calculated as cases/10 000 person-months and 1000 person-years, and specific infection rates per total number of ILI recruited were further calculated as both percentages and cases/100 person-years.
Influenza and RSV incidence rates were also calculated for children with asthma using the same methods as above. The numerator was calculated using recruited children presenting with a new diagnosis of asthma or known to have asthma from the history. The age-band populations were based on an asthma prevalence of 9% [Reference Gupta and Strachan24]. The influenza transmission period spanned 13 and 14 weeks in the respective seasons and the RSV season spanned 24 and 27 weeks in the respective seasons. This accounted for a person-time of 6·23 and 11·77 person-months for influenza and RSV, respectively. Pearson's and Fisher's exact χ2 tests were used to compare differences in the Asian, White and Black ethnic groups. Confidence intervals (CI) were computed assuming the Poisson distribution for incidences data.
RESULTS
Patient characteristics
Over the two seasons there were 7346 clinical episodes that presented with ILI out of a total of 18 415 (40%) paediatric A&E episodes, which were aged <6 years. Complete age and diagnostic data were available for 4593 out of 4628 ILI presenting in the first season (over 31 weeks) and 497 clinical episodes were screened; in 443 recruited children (10%). In the second season complete data were available for 2705 out of 2718 ILI (cases over 24 weeks) and 480 clinical episodes were screened: in 438 recruited children (16%) (some children had multiple attendances). Figure 1 shows a flowchart of the recruitment process.
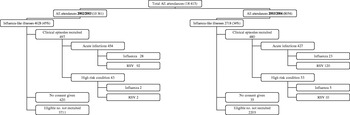
Fig. 1. Flowchart for the recruitment of children into the study.
Table 1 shows baseline comparisons between recruited and non-recruited clinical episodes. Children with recruited episodes were on average 6 months younger (P<0·001) and were 17 times more likely to be admitted than those with non-recruited episodes (P<0·001). There was an overall decrease in the proportion of those with eligible clinical episodes seen in A&E between seasons: 4628 out of 10 361 (45%) during the first season and 2718 out of 8054 (34%) in the second. This decline was mostly due to the large number of the clinical episodes presenting as gastroenteritis during the first season. When gastroenteritis cases were excluded eligible episodes accounted for 35% and 32% in the first and second seasons, respectively. In the study, 86·5% and 81·5% of respective influenza-A- and RSV-infected children resided in Tower Hamlets. As previously stated, 80% of all clinical episodes presenting to the A&E during the study period were aged <72 months and resided in the Tower Hamlets catchment area. There were 455 eligible clinical episodes that were not recruited due to refusal of carer to give consent, non-fluency in English or Bengali of the carer, and social problems. The mean age of this group was 20·4 months, the male:female ratio was 1·65:1 and the in-patient:outpatient ratio was 1:1·2. These clinical episodes were included in the extrapolation exercise as were other non-recruited episodes. There were 56 children aged <1 year and born prematurely. Of these, 20 had RSV and two had influenza.
Table 1. Baseline comparisons between recruited and unrecruited eligible clinical episodes added <72 months seen in hospital A&E departments

Values within parentheses are percentages or 95% confidence intervals.
* Only known for recruited cases.
† Total: No. of clinical episodes, () no. of patients recruited.
Children with high-risk medical conditions for influenza
Underlying high-risk factors were identified in 96 (9·8%) of all children recruited of which seven (7·3%) had influenza, and 12 (12·5%) had RSV identified in their NPA. Of the high-risk cases, 40 children had asthma, 26 congenital heart disease, 21 bronchopulmonary dysplasia, six cystic fibrosis, six sickle cell disease, six were on steroid therapy, five had chronic renal disease, three had thalassaemia, and two suffered from immunodeficiencies. Fourteen children had more than one high-risk condition, (asthma accounted for 42% of all high-risk conditions recruited into the study, i.e. 4·1% of all episodes recruited). Of the children known to have asthma, one had influenza and two had RSV identified in their NPA, although all three did not present with asthma exacerbations.
Virology
Over the two seasons, a total of 315 (33%) viruses were identified in 965 samples taken from children aged <72 months. The viral yield was greater in 2003–2004 than in 2002–2003 (P<0·001, Fisher's exact test): 28% of the samples were positive in 2002–2003 compared with 38% in 2003–2004. The virus-specific rates/100 ILI recruited into the study are shown in Tables 2 a and 2 b.
Table 2(a). Samples (%) detected positive for virus in 2002–2003 (first season): 491 samples tested in children aged <6 years

PCR, Polymerase chain reaction; IF, immunofluorescence; ILI. Influenza-like illness; RSV, respiratory syncytial virus.
Values within parentheses are percentages or 95% confidence intervals.
Table 2(b). Samples (%) detected positive for virus in 2003–2004 (second season): 474 samples tested in children aged <6 years

PCR, Polymerase chain reaction; IF, immunofluorescence; ILI. Influenza-like illness; RSV, respiratory syncytial virus.
Values within parentheses are observed numbers, percentages or 95% confidence intervals.
The overall virus-specific rate/100 ILI for influenza A infections (aggregated 2002–2003 and 2003–2004) (using recruited patients as the denominator) was 11·3/100 person-years (95% CI 8·6–14·6). There was no difference in the virus-specific infection rates/100 ILI cases between the two seasons (P=0·4). RSV infection rates were significantly higher than influenza A infection rates in both seasons. RSV infection rates were higher in 2003–2004 when compared with the 2002–2003 season.
Laboratory-confirmed influenza admissions were seen in Asians (5·5%), Whites (0·9%), Blacks (8·0%) and other groups (4·3%) (χ2=5·6, P=0·03), whereas the A&E outpatient cases were seen in Asians (9·7%), Whites (7·3%), Blacks (7·8%) and other groups (2·8%) (χ2=0·42, P=0·87). Laboratory-confirmed RSV admissions were seen in Asians (23·1%), Whites (29·7%), Blacks (22·7%) and other groups (19·6%) (χ2=2·22, P=0·35), whereas the RSV A&E outpatient cases were seen in Asians (18·9%), Whites (27·3%), Blacks (23·5%) and other groups (16·5%) (χ2=1·95, P=0·37).
Influenza and RSV incidence rates
Extrapolated influenza A and RSV hospital incidence rates in children aged <72 months residing in Tower Hamlets (based on the Tower Hamlets population size) and those seen in A&E during the study period are shown in Tables 3 and 4. Tables 5 and 6 show the influenza A and RSV incidence rates for children aged between 2 and 5 years who were known to have asthma. Table 7 shows the age-specific band influenza and RSV rates/1000 per year for the borough population and hospital population. Figure 2 shows the periods of influenza and RSV circulation by week.
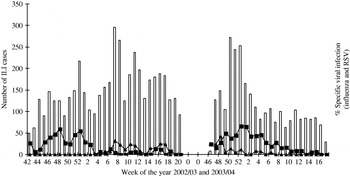
Fig. 2. Weekly influenza-like illnesses (ILI) and specific viruses in 2002–2003 and 2003–2004 winter seasons. □, Weekly number of influenza-like cases; –■–, weekly (%) of respiratory syncytial virus cases; –▲–, weekly (%) of influenza cases recruited.
Table 3. Age-specificinfluenza incidence rates

CI, Confidence interval.
The influenza adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·865 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
Table 4. Age-specific respiratory syncytial virus (RSV) incidence rates

CI, Confidence interval.
The RSV adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·815 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
Table 5. Age-specific influenza incidence rates in children aged 2–5 years with asthma

CI, Confidence interval.
The influenza adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·865 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
The asthma age-band population was based on a prevalence of asthma of 9% in children aged 2–5 years in the United Kingdom.
The definition of a child with asthma for the purpose of the study was a child diagnosed with asthma during the study or a child known to have asthma.
Table 6. Age-specific respiratory syncytial virus (RSV) incidence rates in children aged 2–5 years with asthma

CI, Confidence interval.
The RSV adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·815 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
The asthma age-band population was based on a prevalence of asthma of 9% in children aged 2–5 years in the United Kingdom.
The definition of a child with asthma for the purpose of the study was a child diagnosed with asthma during the study or a child known to have asthma.
Table 7. Burden of influenza A and respiratory syncytial virus (RSV) childhood infections

A&E, Accident and Emergency department.
The no. of influenza and RSV cases per 1000 population is per year (i.e. is divided by a factor of 2).
The <2-year-old influenza hospitalization rate per 1000 is 2·8/1000.
The <2-year-old influenza A&E outpatient rate per 1000 is 1·45/1000.
The number of hospital admissions and A&E attendances are over the winter season (i.e. the study periods).
DISCUSSION
RSV and influenza infections are the two most common viral causes of ILI in childhood in the industrial world but their exact contributions are unknown [Reference Zambon25]. We prospectively studied infants and young children presenting with respiratory illnesses and other influenza-related presentations to the Royal London Hospital paediatric A&E over two winter seasons.
The percentage of laboratory-confirmed influenza in children enrolled into our study was 6% which is similar to the 5·4% estimated for Leicestershire in the 2001–2002 season [Reference Nicholson9], suggesting low influenza transmission over three consecutive seasons in the United Kingdom. These findings are further supported by Health Protection Agency influenza surveillance statistics [Reference Crofts26, Reference Crofts27].
Our influenza hospitalization rates, and laboratory-confirmed influenza percentages were comparable to those documented in the United States [Reference Izurieta1, Reference Neuzil3, Reference Iwane28, Reference Poehling29], Germany [Reference Forster30], France [Reference Ploin8, Reference Aymard, Valette and Luciani31], Italy [Reference Principi32] and South Korea [Reference Kim, Lee and Lee33]. Noticeably, these were lower than those documented in a number of other studies carried out in Spain [Reference Navarro-Mari7], the United States [Reference Glezen and Couch34, Reference Glezen35], Japan [Reference Sugaya36] and Hong Kong [Reference Chiu37]. In contrast, our rates were higher than some studies performed in the United States, Finland and a study performed in the United Kingdom in the late 1970s [Reference Neuzil2, Reference O'Brien4, Reference Martin, Gardner and McQuillin38, Reference Heikkinen39]. Some of these studies may have been carried out during different intensities of influenza transmission when compared to our study. Part of the discrepancy in rates may also be explained by the different methodologies used in some of these studies, as most of the large studies were retrospective and/or ecological studies.
RSV hospitalization rates in infants were comparable to previous studies; 21/1000 infants per year vs. 30–40/1000 infants per year in most studies in Europe and the United States. But lower than that documented in Japan and a US study in Alaskan Native American Indians [Reference Izurieta1, Reference Muller-Pebody10–Reference Saijo14, Reference Lowther40, Reference Clark41]. Our RSV rates were higher than the rates reported in retrospective studies performed in Germany and Sweden [Reference Weigl, Puppe and Schmidt15, Reference Eriksson16].
The age-specific pattern for hospitalization rates was similarly reflected in the RSV A&E outpatient incidence rates where the burden was also highest in the <2 years age group and particularly exaggerated in those aged <1 year, this is similar to findings in other studies [Reference Izurieta1, Reference Eriksson16, Reference Constantopoulos42, Reference Sims43]. Our study also documents that influenza hospitalization rates were highest in the <2 years age group and particularly exaggerated in those aged <6 months. In contrast, the influenza A&E outpatient rates were highest in those who were aged 6–59 months and these patterns were similar to those seen in other prospective studies.
Overall RSV and influenza infections in the <2 years age group accounted for almost 14% and 3% of all hospitalizations and 7% and 2% of all A&E outpatient visits, respectively, supporting the important epidemiological burden of both infections on hospital services.
Of note the overall influenza A&E rates were seven times higher than the hospitalization rates [Reference Iwane28, Reference Aymard, Valette and Luciani31, Reference Sugaya36] and the age-specific A&E outpatient rates peaked in the 2–3 years age group. It has been inferred that pre-school centres play a role in the transmission of influenza. However, in our study population the utilization of these centres was extremely low in the <3 years age group, most children making their first contact with groups at the age of 3–4 years when they first start nursery school. We cannot exclude an element of bias as observed numbers were small and a number of adjustments were made in calculating the incidences but every effort was made to collect robust data.
Interpretation of influenza incidence rates is constrained by under-reporting and this is further inhibited by the impracticability of performing respiratory viral testing on all children presenting with an ILI. Our study suffered to a lesser degree from this problem because of its prospective design and our use of diagnostic knowledge for all those seen in A&E. The criteria for eligible children were based on the fact that most of the specific diagnoses included are associated with influenza. Less common associations such as febrile gastroenteritis were included to enhance the robustness of the results. But the latter did not affect the overall incidence estimates, as the influenza proportion positivity was low in this diagnosis. The recruited children were younger and more likely to be admitted when compared to those unrecruited and therefore the study was open to selection bias in favour of selecting more severe patients because only those admitted to hospital ‘after hours’ were included. Adjustments have been made in the analyses to correct for these biases but an element of bias may remain resulting in an underestimate of the true rate of the diseases as both infections are known to be largely community-based infections.
It has been suggested that ethnicity may play a role in the epidemiology of both influenza and RSV infections. Asian (Bangladeshi) was the most predominant ethnic group in our recruited study population and the highest laboratory-confirmed influenza hospitalization infection proportions were documented in those of Black ethnicity. This was 9·5 and 1·5 times higher than the White and Asian groups, respectively. A study in the United States showed that the influenza hospitalization proportions were higher in Blacks and Hispanics when compared to Whites [Reference Iwane28]. Adjustments were not made for ethnicity but our recruited population was a reflection of the Tower Hamlets borough childhood population. Notwithstanding there may be some limitation for generalizability when applied across the United Kingdom, particularly as regards influenza hospitalization rates. One strength of the study was that it was conducted over two seasons. We note the difference in both the length of the study periods and time of commencement and as a result there may have been some limitation in interpretation of the data. We believe this is minimal as time-frames and commencement of the influenza seasons in both study periods are comparable with the national UK data. A caveat was that entry onto the eligible non-recruits' database was based on the attending clinician's clinical diagnosis, as the research team in the hospital performed no independent evaluation for accuracy and consistency of diagnosis. Analyses for discharge diagnosis of those admitted were based on the final diagnosis.
The current recommendation for paediatric influenza immunization in the United Kingdom is to target clinical risk groups in those aged ⩾6 months. These risk groups comprise children with asthma and other chronic respiratory conditions, haemoglobinopathies, the immunosuppressed, children with chronic cardiac, renal and liver disease, and those with diabetes.
In our study 10% of all the children had underlying high-risk conditions of which almost half had asthma. It is to be noted that influenza and RSV incidence rates in children with asthma were extremely low in our study in contrast to healthy children and similar findings have been seen in other studies conducted in the United Kingdom [Reference Fleming44]. Therefore it can be assumed that our rates are well-matched with those for healthy children. It is not clear why our findings differ from those seen in the United States. Controversy exists as to whether influenza infections trigger asthma exacerbations in children and a number of studies in the United States have supported the role of influenza in causing substantial burden in these children [Reference Izurieta1, Reference Neuzil2, Reference Neuzil45, Reference Mullooly and Barker46]. Christy et al. and Bueving et al. in the United States and The Netherlands respectively demonstrated that influenza vaccination did not reduce the incidence of asthma exacerbations [Reference Christy47, Reference Bueving48]. These latter findings are supported by a Cochrane review on the benefits of influenza vaccination in asthmatics [Reference Cates49]. Unfortunately there was no background data collected to analyse the overall influenza incidence rates in those with other high-risk conditions in our study.
The United States based the expansion of the influenza vaccination programme to cover children aged 6–23 months on evidence that showed that they had influenza hospitalization and outpatient incidence rates similar to those children with high-risk conditions. More recently this has been further expanded to include all children aged <5 years. This is the first comprehensive prospective study in the United Kingdom on the hospital burden of influenza and RSV in childhood, which clearly details the rates of both viruses in the population during two low-intensity transmission seasons. Further studies are required to document these rates in the community and during varying degrees of intensity using the same methodology.
Expanding the current vaccination programme to include healthy children aged <23 months should be considered as hospitalization rates are highest in those aged <2 years. Furthermore, we recommend that all children aged <2 years be immunized against RSV when the ideal vaccine arrives on the market as RSV creates an important burden in the hospital.
ACKNOWLEDGEMENTS
We acknowledge Dr Stephen Teo, Rebecca I'Anson, Shelley Mieres, Ann Bruce and Lynn Hardie for their assistance in the recruitment of children and reviewing the manuscript. We thank the children and parents immensely for taking part in the study. We are also grateful to the medical and nursing staff of the Royal London Hospital.
DECLARATION OF INTEREST
Dr E. K. Ajayi-Obe received partial funding to attend and present at the International Congress on Pediatrics, Mexico 2004. Dr E. David G. McIntosh is employed by Wyeth, one of the manufacturers of influenza vaccines. Professor R. Booy has been a consultant to GSK and Wyeth. The study was funded by an unrestricted grant from Wyeth.














