INTRODUCTION
Streptococcus pneumoniae is a frequent aetiological cause of community-acquired pneumonia (CAP) in children. The 7-valent pneumococcal conjugate vaccine (PCV7), introduced in the USA and Europe, has reduced the incidence of invasive pneumococcal disease (IPD) caused by vaccine serotypes (serotypes 4, 6B, 9V, 14, 18C, 19F, 23F) and of carriage of these serotypes [Reference Pilishvili1–Reference Bettinger4]. Several reports indicate that PCVs are effective against pneumonia [Reference Black5–Reference Madhi, Klugman and Group7]. Black et al. [Reference Black5] reported that PCV7 reduces the incidence of first episode of clinically diagnosed pneumonia by 6·0%. The rate of all-cause pneumonia hospitalizations in children in the USA aged <2 years decreased by about 35% after the vaccine was licensed [Reference Grijalva6]. In these studies, bacteraemic pneumococcal pneumonia constituted a minority of the total amount of observed clinical pneumonia. These results indicate that PCV not only prevents invasive pneumococcal pneumonia but also reduces the incidence of all-cause pneumonia. However, little is known about the rate of pneumonia attributable to S. pneumoniae and their serotypes.
PCV7 was introduced in Japan in February 2010. Surveillance of the population-based incidence of CAP and molecular characterization of isolates causing CAP are fundamental to understanding the impact of PCV7 and to assessing whether the genetic structure of the pneumococcal population changes after implementation of an immunization programme. However population-based studies of CAP in Japanese children are rare.
To estimate bacterial pathogens and to better manage lower respiratory tract infections in children, we examined microbiological specimens of washed sputum according to the Japanese Guidelines for the Management of Respiratory Infectious Diseases [Reference Kubo8–Reference Uehara13]. Here, we surveyed the incidence of CAP in hospitalized children to obtain baseline data before the introduction of PCV7. We also determined the isolation rate of S. pneumoniae in children with CAP using washed sputum and blood samples to estimate the effect of PCV7 on CAP. The isolates were characterized by serotyping and by multilocus sequence typing (MLST).
METHODS
Incidence of CAP and of CAP with pneumococcal bacteraemia in Chiba City
We determined the annual incidence of hospitalized CAP and CAP with pneumococcal bacteraemia in children aged <16 years in Chiba City as follows. We retrospectively counted the total number of patients admitted to 18 hospitals with paediatric wards in and around Chiba City serving the catchment population between 1 April 2008 and 31 March 2009. A questionnaire was sent to 18 hospitals and information was obtained from the clinical records of all of them. We defined CAP as pneumonia that occurred in patients who had not been hospitalized within the past 2 weeks. Acute lower respiratory infection was diagnosed by clinicians at each hospital based on clinical symptoms of one or more of: fever, rapid or difficult breathing, cough and crackle in lung fields on auscultation. Radiographs were taken before admission and the diagnosis of CAP was confirmed by clinicians based on positive radiograph findings at the time of occurrence. Patients with CAP who did not require hospitalization were excluded from this study. The catchment area comprised 944 557 inhabitants, including 140 345 and 42 606 children aged <16 and <5 years, respectively [14].
Rate of S. pneumoniae isolated from sputum and blood
We surveyed children who were admitted to six major hospitals in Chiba City. These six hospitals covered 75% of hospitalized children who were diagnosed with CAP within the city during 2005. [Reference Ogita15]. Written informed consent was obtained from the parents or guardians of the patients before collecting samples, in accordance with the guidelines of the Institutional Review Board of Chiba University. Demographic and clinical data were collected by paediatricians. Upon admission, blood samples were collected and sputum samples were obtained using a tongue depressor with a light as follows. The tongue was depressed to induce the cough reflex and then sputum was collected using a swab or aspirated into a 1-ml disposable syringe. Sputum samples were washed three times in sterilized saline as described previously [Reference Uehara9]. A small portion of washed sputum was homogenized and smeared onto glass slides for Gram staining. Stained smears were judged valid according to Geckler's classification based on the number of leucocytes or alveolar macrophages and squamous or ciliated epithelial cells per low-power field (100x). Smears with Geckler's groups of 4–5 containing >25 leucocytes or macrophages and <25 squamous or ciliated epithelial cells in the low-power microscopic field (100x) were considered adequate. Washed sputum and blood samples were cultured at the microbiology laboratory of each hospital. Pathogens accounting for >50% of the colonies in culture or presenting >1×107c.f.u./ml of washed sputum were regarded as ‘dominant’. S. pneumoniae isolates dominant in sputum samples and/or isolates from blood samples were initially stored at −80°C at each hospital and then sent to Chiba University Hospital and the Department of Bacteriology of the National Institute of Infectious Diseases.
Antimicrobial susceptibility
Antimicrobial susceptibility was tested in vitro using broth dilution according to the Clinical and Laboratory Standards Institute guidelines (CLSI M100-S18). Although the CLSI published new breakpoints for penicillin therapy in 2008 (CLSI M100-S18), we used the previously published breakpoints. S. pneumoniae was interpreted as susceptible (PSSP), intermediate (PISP), and resistant (PRSP) if the minimum inhibitory concentration (MIC) of penicillin G was ⩽0·06, 0·12–1 and ⩾2 μg/ml, respectively.
Serotyping
Serotypes were determined by the Quellung reaction using antiserum purchased from Statens Serum Institut, Copenhagen, Denmark. We serotyped 6C and 6D using an in-house antiserum and confirmed the results by genetic characterization as described previously [Reference Chang16].
MLST
We performed MLST as described previously [Reference Enright and Spratt17]. Briefly, internal fragments of each of the seven housekeeping genes, aroE, gdh, gki, recP, spi, xpt and ddl were amplified by Polymerase chain reaction (PCR) and their sequence types (STs) were determined by reference to the MLST database (http://spneumoniae.mlst.net/). New alleles and allelic profiles were submitted to the database for assignment. The relatedness of isolates and known similar strains in the database were determined by constructing a neighbour-joining tree using the online program, Draw Tree Using Own MLST Data. Relationships among the isolates were determined using eBURST v. 3 software and strains were assigned to clonal complexes (CCs) using the definition of a stringent group, in which all STs share six of seven identical alleles with at least one of the other STs within the group [single-locus variants (SLV)]. We compared STs with those of 43 pneumococcal clones in the Pneumococcal Molecular Epidemiology Network (PMEN; http://www.sph.emory.edu/PMEN/).
Statistical analysis
Data were analysed using PASW Statistics 18 (SPSS Japan Inc., Japan). Associations between underlying diseases and the number of hospitalizations or the results of sputum culture were tested using Fisher's exact test. Correlations between age and isolation rates of pneumococcus and coverage rates of PCV7 were analysed using Pearson's χ2 test. A P value of <0·05 was considered statistically significant.
RESULTS
Annual incidence of CAP
During the study period, CAP caused 860 episodes of children being hospitalized. The incidences of CAP in children aged <16 and <5 years were 6·13 and 17·6/1000 child-years, respectively.
Annual incidence of CAP with pneumococcal bacteraemia
Five patients were diagnosed with pneumococcal bacteraemia combined with CAP. The incidences of CAP with pneumococcal bacteraemia in children aged <16 and <5 years were 3·56 and 11·7 episodes/100 000 child-years, respectively.
Isolation of S. pneumoniae from sputum and blood culture
A total of 579 children with 626 episodes were admitted to six major hospitals with a diagnosis of CAP. This corresponded to 73% of all CAP episodes occurring in Chiba City during the study period. We obtained sputum and blood samples representing 502 (80·2%) and 544 (86·9%) of the 626 episodes. S. pneumoniae was identified and culture-dominant in 175 (27·9%) and 92 (14·7%) sputum samples, respectively. Of five patients with blood samples that were positive for S. pneumoniae, one also had a positive sputum culture. The serotypes and STs of the blood and sputum isolates from this patient were identical (serotype 6B, ST90).
Figure 1 shows the age distribution of children with CAP and the results of S. pneumoniae identified in sputum and blood cultures. The median age of children with CAP episodes was 1 year. Patients with CAP included 331 (52·9%) children aged <2 years and 230 (36·7%) aged 2–4 years. The median age of children with pneumococcus-positive episodes was also 1 year. S. pneumoniae was isolated from the blood of one 2-year-old and four 1-year-old patients. The detection rate of pneumococcus from sputum was the highest in children aged 2–4 years (18·7%), followed by those aged <2 (11·5%) and 5–15 (16·9%) years [statistically not significant, χ2(2)=5·92, P=0·052].
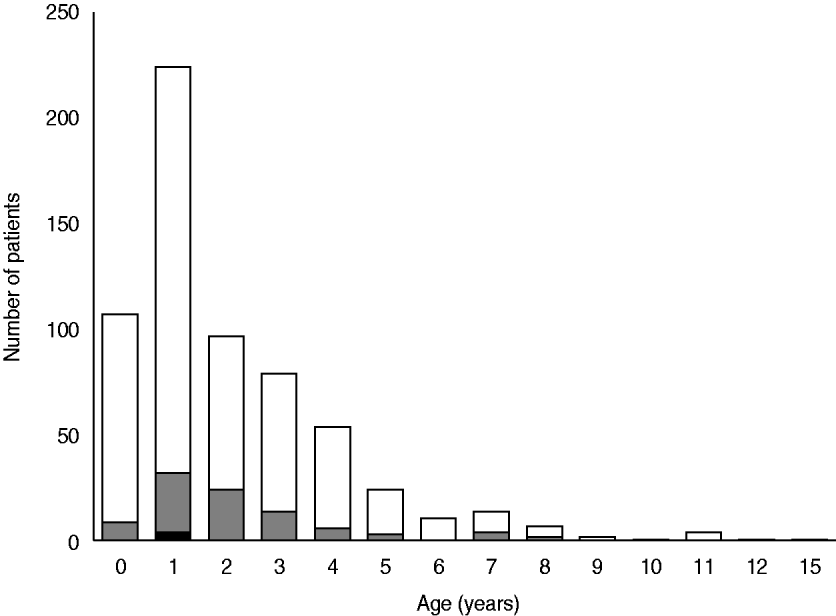
Fig. 1. Age distribution and identification of Streptococcus pneumoniae in children hospitalized with community-acquired pneumonia. ![]() , S. pneumoniae dominantly isolated from sputum; ▪, S. pneumoniae isolated from blood (n=626).
, S. pneumoniae dominantly isolated from sputum; ▪, S. pneumoniae isolated from blood (n=626).
Of the 579 patients, 215 had one or more underlying diseases, 174 had bronchial asthma, 24 were premature, ten had congenital heart diseases, seven had chromosome anomalies, five had cerebral palsy, and 18 had other diseases. Thirty-seven patients were hospitalized more than once. Multiple hospitalizations were significantly associated with bronchial asthma (P<0·001), congenital heart disease (P=0·021) and cerebral palsy (P=0·035) (Table 1). Underlying diseases and the detection of S. pneumoniae from sputum were not significantly related. No one had sequelae with CAP episodes.
Table 1. Risk of multiple hospitalizations with community-acquired pneumonia based on underlying diseases
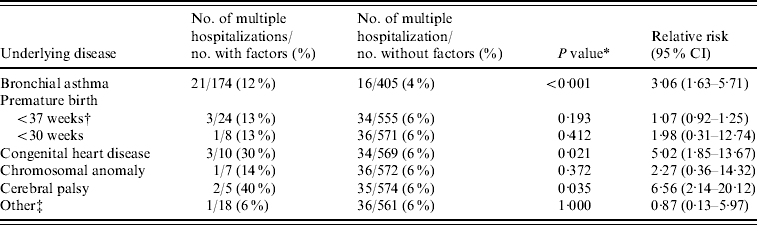
CI, Confidence interval.
* Fisher's exact test.
† Including preterm birth <30 weeks.
‡ Epilepsy (2), neutropenia (2), achondroplasia (1), acute myeloid leukaemia (during consolidation therapy) (1), bronchiectasis (1), congenital diaphragmatic hernia (1), cretinism (1), gastro-oesophageal reflux disease (1), Kawasaki disease (1), mitochondrial diseases (1), periodic fever syndrome (1), polycystic kidney disease (1), post-cleft lip and palate repair (1), Sotos syndrome (1), tracheal stenosis (1), malnutrition (1).
Antimicrobial susceptibility and serotype distribution
Antimicrobial susceptibility and serotypes were tested against 63/92 cultured sputum isolates in which S. pneumoniae was the dominant organism and against five blood isolates. Table 2 shows the susceptibility of the S. pneumoniae isolates to penicillin G and their serotype distribution. The rates of PISP and PRSP in sputum isolates were 54% and 22%, respectively, and 40% and 40%, respectively, in the blood isolates. Resistance rates in sputum isolates against cefotaxime (MIC ⩾2 μg/ml), erythromycin (MIC ⩾1 μg/ml) and clindamycin (MIC ⩾1 μg/ml) were 4·8%, 92% and 60%, respectively, and 20%, 100% and 60%, respectively, in blood isolates. All isolates were susceptible to meropenem and vancomycin.
Table 2. Serotype distribution and susceptibility of Streptococcus pneumoniae isolated from samples obtained from children with community-acquired pneumonia in Japan
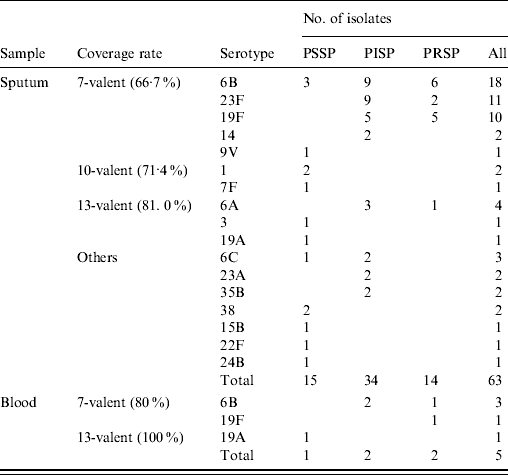
PSSP, Penicillin-susceptible S. pneumoniae; PISP, penicillin-intermediate S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
Of the 17 identified serotypes, the most frequent in the sputum isolates were 6B (28·6%), 23F (17·5%), and 19F (15·9%) and those in the blood isolates were 6B (60%), 19F (20%), and 19A (20%). Serotype 6C was identified in three sputum isolates. Serotype 6D was not found. The coverage rates of PCV7 were 66·7% and 80·0% in sputum and blood isolates, respectively. The coverage rates in sputum isolates based on age were 65·2%, 73·5% (70·2% in those aged <5 years) and 33·3% in children aged <2, 2–4 and 5–15 years [statistically not significant, χ2(2)=3·74, p=0·154]. Ten-valent (PCV7 plus additional serotypes 1, 5, 7F), 13-valent (PCV7 plus additional serotypes 1, 3, 5, 6A, 7F. 19A) and investigational 15-valent (PCV7 plus additional serotypes 1, 3, 5, 6A, 7F, 19A, 22F, 33F) PCVs would potentially increase the coverage rates of sputum isolates by 4·7%, 14·3% and 15·8%, respectively. The 13-valent and investigational 15-valent PCVs covered all of the blood isolates.
The serotypes of PSSP in sputum isolates widely varied whereas the 14 PRSP sputum isolates fell into only the following serotypes: 6B (42·9%), 19F (35·7%), 23F (14·3%) and 6A (7·1%). The PISP sputum isolates were represented by eight serotypes with 6B (26·5%), 23F (26·5%), 19F (14·7%) and 6A (8·8%) being the most prevalent. The serotypes of PRSP in blood isolates were also 6B (50%) and 19F (50%), whereas that of PISP isolates was only 6B (100%). The PCV7 and PCV13 coverage rates for PRSP were 92·9% and 100% in sputum, respectively, and 100% in blood isolates.
MLST
MLST was performed on 61/92 sputum isolates in which S. pneumoniae was the dominant organism and on five blood isolates. Of the 66 isolates, 37 STs were found including nine new STs (ST5830–5834 and ST5494–5497) with four new alleles. A dendrogram was constructed (Fig. 2) and eBURST analysis revealed six CCs and 25 singletons containing 23 and 43 isolates, respectively. Furthermore, 54·1% and 40% of the sputum and blood isolates had STs identical to 11 international PMEN clones or their SLVs. Eight multidrug-resistant PMEN clones comprised Spain6B-2, Taiwan19F-14, Taiwan23F-15, Spain23F-1, Utah35B-24, Colombia23F-26, Portugal19F-21, Greece6B-22 and three susceptible PMEN clones comprised Sweden1-28, Netherlands15B-37 and Netherlands7F-39. Isolates related to the eight multidrug-resistant PMEN clones comprised 49·1% and 40% of sputum and blood isolates, respectively. Table 3 shows the numbers and antimicrobial susceptibility of isolates with STs identical to multidrug-resistant PMEN clones or their SLVs. Sputum isolates related to multidrug-resistant PMEN clones comprised 62·5% and 71·4% of PISP and PRSP clones, respectively.
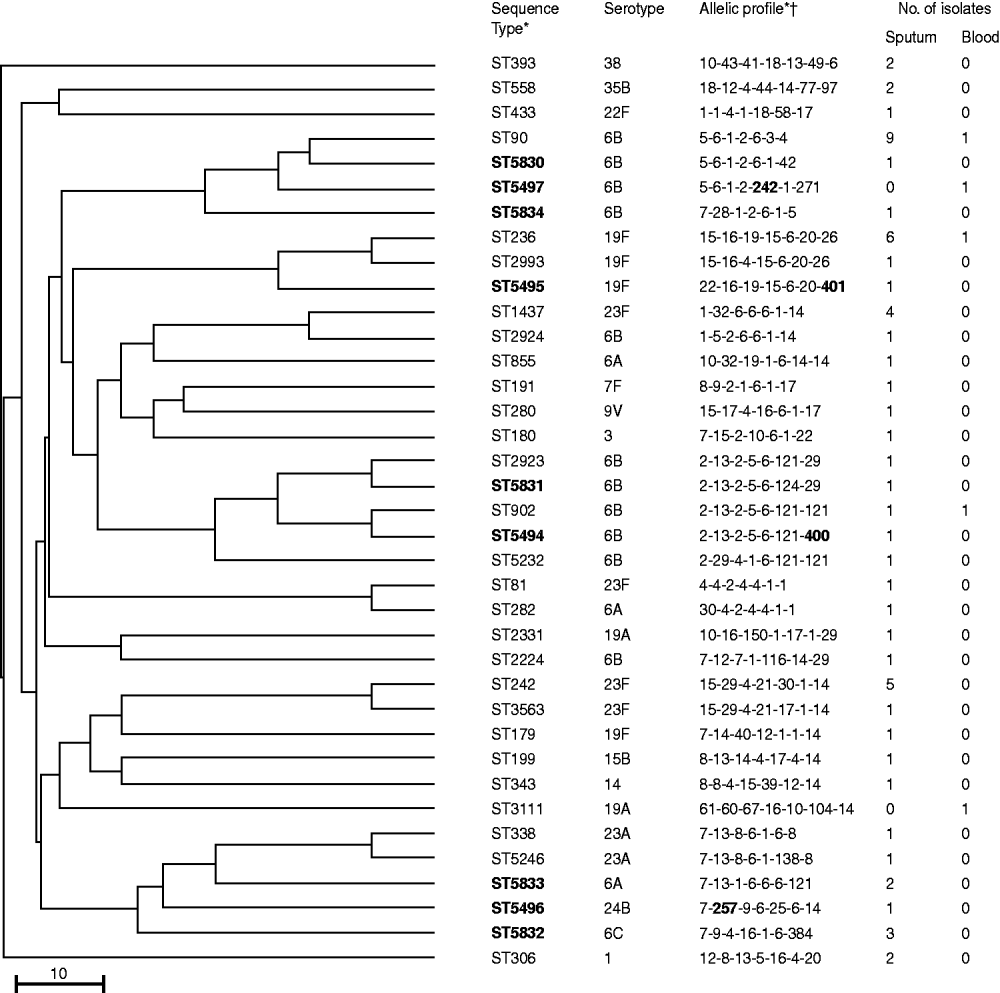
Fig. 2. Genetic relatedness, multilocus sequence-typing profile, and serotypes in 37 sequence types of 66 Streptococcus pneumoniae isolates from children with community-acquired pneumonia in Japan. Scale bar indicates genetic linkage distance. PMEN, Pneumococcal Molecular Epidemiology Network. * New sequence types and alleles in bold. † In the order: aroE-gdh-gki-recP-spi-xpt-ddl.
Table 3. Antimicrobial susceptibility of isolates with sequence types identical to multidrug-resistant PMEN clones or their single locus variants
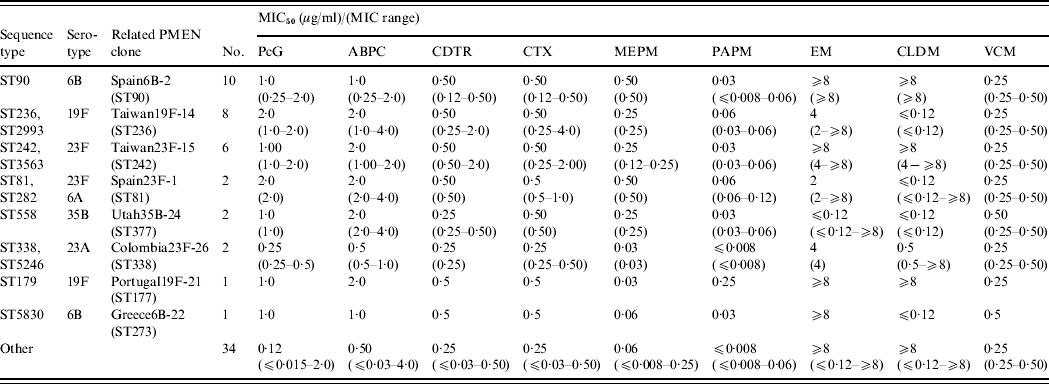
ABPC, Ampicillin; CDTR, cefditoren; CLDM, clindamycin; CTX, cefotaxime; EM, erythromycin; MEPM, meropenem; MIC, minimum inhibitory concentration; PAPM, panipenem; PcG, penicillin G; PMEN, Pneumococcal Molecular Epidemiology Network; VCM, vancomycin.
DISCUSSION
The annual incidence of CAP in children aged <5 years who were hospitalized with this condition was 17·6 episodes/1000 child-years and that of CAP with pneumococcal bacteraemia in those aged <5 years was 11·7 episodes/100 000 child-years. In 626 episodes, S. pneumoniae was dominant in 14·7% and 0·8% of sputum and blood samples, respectively.
Our findings of the incidence of pneumococcal bacteraemia in children hospitalized with CAP were equivalent to those of our previous survey [Reference Ogita15, Reference Ishiwada18]. The incidence of pneumonia in children aged <5 years that required hospitalization before the introduction of PCV7 in the USA and European countries was 2·25–6·55/1000 child-years [Reference Clark19–Reference Henrickson21]. The annual incidence of paediatric CAP was higher in the present study than in these reports and similar to that of a study in Germany that included outpatients (13·7–16·9/1000 child-years in children aged <5 years) [Reference Weigl22]. The reasons for the variation in incidence rates in countries might include differences in access to healthcare, willingness to hospitalize patients and the costs of admission. Free access to any hospital or clinic is guaranteed in Japan, and the costs of almost all medical care for children up to age 6 years in Chiba City are compensated by local government. Thus, most children with CAP in Chiba City, and infants in particular, were treated in hospital.
Another possible reason for the higher incidence is differences in the definition of pneumonia. Pneumonia is usually diagnosed in Japan based on clinical signs and chest radiographic findings confirmed by clinicians, not radiologists. A World Health Organization (WHO) working group developed a method for standardizing the interpretation of chest X-rays of children for epidemiological purposes [Reference Cherian23]. Pneumonia was diagnosed by clinicians at each hospital in the present study and included not only WHO-confirmed end-point pneumonia but also other radiographic findings. The incidence of CAP with end-point pneumonia according to WHO standards has yet to be determined.
Identifying the aetiology of childhood CAP is difficult because of the lack of accurate, non-invasive tests. The diagnostic yields of sputum culture are limited by potential contamination from the upper respiratory tract. However, the reliability of microbiological sputum tests can be improved by washing. Bartlett & Finegold [Reference Bartlett and Finegold24] showed that washing sputum decreases the number of contaminants by 100- to 1000-fold and does not result in a qualitative and quantitative loss of bacteria recovered in percutaneous transtracheal aspirates. Combining quantitative culture with washing sputum specimens enhances the value of findings. We combine a washing technique with semi-quantitative culture to evaluate pathogenic bacteria [Reference Kubo8, Reference Uehara9]. We applied this method with serology to determine the aetiology of CAP in 596 hospitalized children between 1990 and 1991 [Reference Ishiwada25] and identified pathogens in 64·4% of them. Evidence of bacterial, Mycoplasma pneumoniae and viral (mostly respiratory syncytial virus) infection was found in 28·8%, 14·9% and 29·9%, respectively, of these children. Two major bacterial pathogens were Haemophilus influenzae (19·6%) and S. pneumonia (8·6%). The major pathogens defined in this study were consistent with a study of 1700 Japanese paediatric patients with CAP using real-time reverse transcription–PCR [Reference Hamano-Hasegawa26]. Moreover, the clinical responses to antibiotics administered based on the results of sputum culture are good [Reference Cao10, Reference Takeda11].
Of the 626 episodes in six hospitals examined in the present study, S. pneumoniae was identified as the causative pathogen in 96 (15·3%) episodes. Five and 92 were identified from blood and sputum cultures, respectively, including one that tested positive in both cultures. The rate of infection with pneumococcus (15·3%) was similar to that described in a study from Turkey (17·1%) that used washing and quantitative sputum cultures [Reference Ziyade, Aksu and Yagci27], and with a study from Italy (17·8%) that used serological assays with paired sera [Reference Don28]. However, the findings were relatively lower than those in a study from the USA using pneumolysin-based PCR (44%) [Reference Michelow29], even when all S. pneumoniae isolates identified in sputum (27·9%) were taken into account. One of the limitations of the present study is the absence of information about previous antibiotic use. The low rate of S. pneumoniae detection herein compared with PCR might be related to previous use of antibiotics, which is frequent in Japan.
We could not identify a relationship between underlying disease and the detection of S. pneumoniae. However, patients with asthma, congenital heart disease, and cerebral palsy had multiple hospitalizations for CAP. The only vaccines for the prevention of bacterial pneumonia (excluding pertussis) are H. influenzae type b and pneumococcal vaccines. Therefore, such patients should be recommended for immunization with these vaccines, both of which are elective in Japan.
The incidence of S. pneumoniae that is not susceptible to penicillin has rapidly increased in Japan since around 1990 [Reference Yoshida30]. The rate of PRSP in the present study was as high as that in previous studies of IPD [Reference Ishiwada18] and acute otitis media (AOM) [Reference Hotomi31] in Japanese children. The frequency of STs related to multi-resistant PMEN clones was also high in the present study. The spread of these clones might be responsible for the high rate of resistant strains developing in Japanese children.
The most prevalent serotype in sputum isolates of children with CAP was 6B, followed by 19F and 23F. The high prevalence of these serotypes was the same as that in a report describing IPD in Japanese children [Reference Chiba32]. The overall PCV7 coverage rates in sputum and blood were 66·7% and 80%, respectively. These rates in children aged <5 years were 70·2% and 80%, respectively. PCV7 coverage of bacteraemic pneumonia was equal to that of IPD in the USA and Europe before the introduction of PCVs [Reference Hausdorff, Feikin and Klugman33]. However, serotype 14, which is the most common serotype in the USA and Europe preceding PCVs [Reference Hausdorff34], and serotype 1, which has been predominant in complicating pneumonia before and after PCVs became available [Reference Hausdorff and Dagan35], were undetectable in our blood culture. Information about non-bacteraemic pneumonia serotypes is limited. The coverage rates of PCV7 in patients aged <5 years with respiratory infections determined using oropharyngeal swab samples in Vietnam, hypopharyngeal aspirates in China and nasopharyngeal isolates in Switzerland were 88·7% [Reference Watanabe36], 76·3% [Reference Yao37], and about 70% (<2 years) [Reference Muhlemann38], respectively, with serotype 19F being the most frequent.
PCV7 coverage rates for PRSP were 92·9% and 100% in sputum and blood isolates, respectively. We hope that PCV7 will reduce the incidence of respiratory tract infections, especially those caused by strains that are less susceptible to penicillin.
Serotypes have changed in countries where PCV7 has been introduced as routine immunization and the emergence of serotype 19A with multidrug resistance has become a problem [Reference Pilishvili1, Reference Isaacman, McIntosh and Reinert2, Reference Moore39–Reference Choi41]. ST199 and ST320 are the major STs found in these countries. Here, we found only serotype 19A S. pneumoniae with ST2331. One isolate had ST199 but its serotype was 15B.
The incidence of serotype 6C, which was distinguished from serotype 6A in 2007 [Reference Park42], also increased after the introduction of PCV7 [Reference Tocheva43]. Serotype 6C was isolated from <2% of children with IPD and from 9·5% of samples from the nasopharyngeal mucosa of healthy children in Japan [Reference Chang16]. We identified three 6C isolates from sputum (4·8%) with the new sequence type ST5832 in our patients with CAP.
Some limitations of this study should be considered. This study covered only a 1-year period and therefore does not account for annual variations in either the incidence of disease or the detected serotypes. In addition, information about previous antibiotic administration was not available.
PCV7 was introduced as an elective vaccine in Japan in February 2010. New PCVs, especially 13-valent and the investigational 15-valent types, would potentially increase the coverage rate of sputum isolates. Switching to these new PCVs should be considered with the increase of non-vaccine serotype. Continued surveillance to detect changes in the incidence of CAP caused by pneumococci, their antimicrobial resistance, serotypes and genotypes are crucial for evaluating the impact of PCV7 and to effectively prevent pneumococcal infections.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the assistance of the participants in the Chiba Paediatric Infectious Disease Meeting, namely Fumie Nagai, Masanaga Arima, Katuaki Abe, Hisae Kazukawa, Chie Fukasawa, Tadashi Hoshino, Jiro Aizawa, Hiroko Oshima, Nobuyasu Ishikawa and Yoshio Kori in the planning and implementation of this study. The authors are also grateful to the paediatricians in and around Chiba City who contributed toward this study. This study was financially supported by the Japanese Ministry of Health, Labour and Welfare grants for Research on the Mechanism, Epidemiology, Prevention and Control of Acute Respiratory Infections (H22-iyaku-shitei-028), Research on Regulatory Science of Pharmaceuticals and Medical Devices (H22-kokui-shitei-009) and the Research Project for Emerging and Re-emerging Infectious Disease (H21-shinkou-ippan-008 to A.W.).
DECLARATION OF INTEREST
None.









