INTRODUCTION
National trends in the United States have shown a marked increase in ambulatory visits and hospitalizations for skin and soft-tissue infections (SSTIs) coinciding with the emergence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA), which is the primary cause of up to 64% of SSTIs seen in emergency departments (EDs) nationwide [Reference Frazee1–Reference Zetola4]. More than 90% of SSTIs are treated in the outpatient setting [Reference Miller5]. Among the estimated 34·8 million outpatient visits for SSTIs between 2005 and 2011, about 33% of these were seen in the ED [Reference Taira6–Reference May, Mullins and Pines8].
Up to 70% of SSTI medical visits are complicated by recurrent infections [Reference Graber9–Reference Crum-Cianflone, Weekes and Bavaro12]. In non-ED settings and small cohort studies, risk factors for SSTI recurrence include MRSA aetiology, recent hospitalization, recent SSTI, cephalosporin use, comorbidities (e.g. diabetes, obesity, HIV), and geographical location [Reference Crum-Cianflone, Weekes and Bavaro12–Reference May16]. While there is a growing literature on predictors of recurrent CA-MRSA and S. aureus SSTIs, there are limited data on sociodemographic characteristics and overall predictors of recurrent SSTIs from large cohorts for those patients presenting to the ED for SSTI care.
Improved knowledge of SSTI recurrence epidemiology and risk factors in patients who present to ED settings is critical for informing treatment guidelines, and for guiding future study of ED-based interventions, particularly for high-risk patients. Such knowledge may assist clinicians identify patients at high risk for visits to the ED for recurrent infection. Here we describe rates and predictors of visits to the ED for recurrent SSTIs in a very large population of patients visiting the ED for an SSTI.
MATERIALS AND METHODS
Study design, setting, and data collection
We performed a retrospective study of recurrent SSTIs in patients who visited an ED in California between 2005 and 2011. California, with more than 37 million persons (in 2010), comprises over 12% of the US population, the highest population of any US state, with a large proportion of publicly and underinsured patients and higher than national proportion of patients that use the ED for care [17–Reference Hing and Rui19]. Besides the large size, we chose California because of the ability to track readmissions across facilities within the entire state, the amount of clinical information encoded in its health records, and because it was one of the earliest spots in the United States to be affected by the CA-MRSA epidemic [Reference Moran3, 20–Reference Pan22].
The study utilized California ED discharge data from the State Emergency Department Databases (SEDD) and the State Inpatient Databases (SID) from the Healthcare Cost and Utilization Project (HCUP) of the US Department of Health and Human Services Agency for Healthcare Research and Quality. The dataset included all ED visits and subsequent hospitalizations at non-federal (e.g. non-military, Veterans Administration, or Indian Health Service), short-term general, and other speciality hospitals in California between 2005 and 2011
Statistical methods
We used multivariable logistic regression to investigate the magnitude to which sociodemographic factors and comorbidities at the time of hospitalization were associated with the odds of having one or more recurrent SSTI visits. Variables were selected based on the aetiology of SSTIs, comorbidities associated with an increased risk of SSTIs, and sociodemographic characteristics associated with higher risk of recurring SSTIs based on review of the prior literature [Reference Shastry, Rahimian and Lascher10, Reference Crum-Cianflone, Weekes and Bavaro12, Reference Sreeramoju13, Reference Miller23]. We examined the correlation between variables using Spearman's correlation coefficient, and we assessed multicollinearity using the variance inflation factor. Additionally, a proportional trend analysis was conducted to determine if the rate of SSTI recurrent visits changed over time. Stata v. 14.1 (StataCorp, USA) was used for all analyses.
The analysis included all patients with a principal diagnosis of uncomplicated SSTI or ‘other skin and subcutaneous infections’, using previously described definitions [Reference Miller5]. The included ICD-9-CM codes are listed in Table 1. A recurrent visit was defined as any patient who returned to the ED for an SSTI between 2 weeks and 6 months (180 days) after an initial visit. This conservative time-frame allowed a focus on recurrent cases proximal to the initial SSTI while excluding follow-up visits that were likely for the same infection.
Table 1. Types of skin and soft tissue infections

Data on primary visits were included for years 2006–2010. Primary visit data from years 2005 and 2011 were excluded from the final analysis because recurrent visits within 180 days were not accurately represented for patients whose first visit may have fallen before January 2005 or whose recurrent visit may have fallen after December 2011. Data from 2005 were used only to determine if a primary visit in 2006 was indeed a primary visit and not a recurrent visit from 2005. Data from 2011 were used only for finding recurrent infections.
A number of sociodemographic and patient-level factors were investigated in the model. Sociodemographic factors included age, gender, race, health insurance type, household income, geographical location, degree of urbanization, and visit year. Age was divided into four categories: <15, 15–44, 45–64, and >64 years. Household income was represented by the quartile of the median household income in the patient's zip code. Geographical location was defined as southern and northern California, divided near the 37° latitude (as per the 2010 county-level census data). Degree of urbanization was divided into four categories using the 2003 urban influence codes: large metropolitan area (⩾1 million residents); small metropolitan area (<1 million residents); micropolitan area; and neither metropolitan nor micropolitan area [24].
Patient-level factors included patient discharge disposition (whether the patient was admitted to the hospital after their ED visit), comorbidities, SSTI treatment factors, including antibiotic injection and incision or drainage, and aspiration. Comorbidities investigated in the model included AIDS; alcohol abuse, drug abuse, and liver cancer; breast cancer, lymph cancer, and solid tumour; chronic pulmonary disorder; diabetes; obesity; and peripheral vascular disorder. In addition, a Charlson comorbidity score [Reference Charlson25] was calculated for each patient.
RESULTS
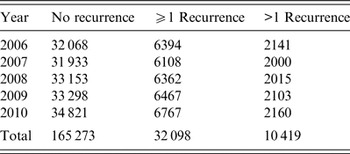
Of 197 371 SSTI patients included in this analysis, 32 098 (16·3%) had at least one recurrent SSTI visit and 10 419 (5·3%) had more than one recurrent SSTI visit (Table 2). SSTI recurrent visit rate varied by year, with the highest recurrent visit rate in 2006 (6394 patients, 16·6%) and the lowest in 2007 (6108 patients, 16·1%). The overall number of SSTI patients increased slightly over this period from 38 462 patients in 2006 to 41 588 patients in 2010 (Table 2). The most common diagnosis was cellulitis, which accounted for 96·75% of visits (ICD-9 codes 681 and 682), although recurrence rates did not differ for different diagnoses (Table 1).
Table 2. Skin and soft tissue infection recurrence by year
The demographic predictors included in this analysis were all significantly associated with a patient's odds of having a recurrent ED visit (Table 3). Non-senior adults were more likely than children to have a recurrent SSTI visit [age 18–44 years: odds ratio (OR) 1·36, 95% confidence interval (CI) 1·27–1·45; age 45–64 years: OR 1·19, 95% CI 1·11–1·27), while the elderly were less likely to have a recurrent visit (OR 0·78, 95% CI 0·72–0·84). Female patients had 11% higher odds of a recurrent visit compared to male patients (OR 1·11, 95% CI 1·08–1·14), and non-Hispanic white patients also had significantly higher rates of recurrent visits than other races/ethnicities (Black: OR 0·87, 95% CI 0·83–0·91; Hispanic: OR 0·90, 95% CI 0·87–0·93; Asian or Pacific Islander: OR 0·61, 95% CI 0·55–0·67).
Table 3. Predictors of skin and soft tissue infection recurrence
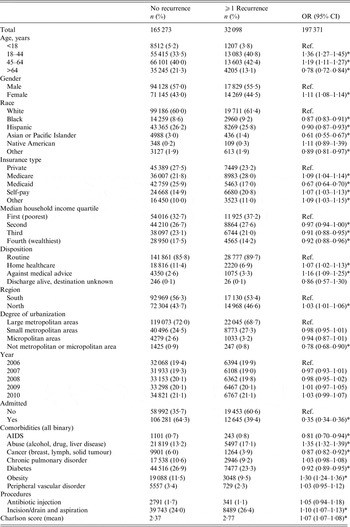
OR, Odds ratio; CI, confidence interval.
* Significant at the 0·05 level.
Insurance status was significantly associated with a patient's odds of a recurrent visit. However, while Medicare coverage or self-pay status were both slightly higher compared to patients with private insurance coverage (Medicare: OR 1·09, 95% CI 1·04–1·13; self-pay: OR 1·07, 95% CI 1·03–1·13), patients with Medicaid were less likely to have a recurrent visit (OR 0·67, 95% CI 0·64–0·70). Geography and income were also important. Patients from wealthier areas were less likely to have recurrent visits (wealthiest vs. poorest quartile: OR 0·92, 95% CI 0·88–0·96). Patients treated in northern California were slightly more likely to have a recurrent visit compared to patients in southern California (OR 1·03, 95% CI 1·01–1·06). In addition, patients treated in rural areas (neither metropolitan nor micropolitan) had 22% lower odds of a recurrent visit compared to patients living in metropolitan areas (OR 0·78, 95% CI 0·68–0·90).
A patient's health condition was significantly associated with the odds of recurrent visits. Admitted patients, who are likely sicker than discharged patients, had 65% lower odds of a recurrent visit compared to patients who were not admitted (OR 0·35, 95% CI 0·34–0·36), however, those who were discharged to home healthcare had higher odds of recurrence (OR 1·07, 95% CI 1·07–1·08). Patients’ odds of a recurrent visit increased by 7% with each 1-unit increase in Charlson comorbidity score (OR 1·07, 95% CI 1·07, 1·08). However, not all of the individual comorbidities analysed were associated with higher odds of a recurrent visit. For instance, patients with a history of drug or alcohol abuse or liver disease had a higher odds of a recurrent visit (OR 1·35, 95% CI 1·32–1·39). Similarly, obese patients had 30% higher odds of a recurrent visit (OR 1·30, 95% CI 1·24–1·36). However, patients with AIDS, cancer, and diabetes all had lower odds of recurrent visits compared to patients without these conditions (AIDS: OR 0·81, 95% CI 0·70–0·94); cancer: OR 0·87, 95% CI 0·82–0·92; diabetes: OR 0·92, 95% CI 0·89–0·95). Moreover, certain comorbidities, such as chronic pulmonary disorder and peripheral vascular disorder were not associated with a patient's likelihood of an SSTI recurrent visit (Table 3).
Treatment (or lack thereof) also impacted the likelihood of a recurrent visit. Patients whose infections were drained or aspirated had higher odds of a recurrent visit compared to patients who did not receive these treatments (OR 1·10, 95% CI 1·07–1·13). Patients who were discharged against medical advice were also more likely to have an SSTI recurrent visit (OR 1·16, 95% CI 1·09–1·25). No significant difference in the odds of an SSTI recurrent visit between the years included in this analysis was found, and a proportional trend analysis also showed no significant trend in recurrent visits over time (χ 2 trend = 0·68, P = 0·409).
Many of the predictors included in this analysis were also significantly associated with a patient's odds of having more than one recurrent visit (Supplementary Table S1). In general, the magnitude of association was greater when predicting multiple recurrent visits than one or more recurrent visits. For example, adults aged 18–44 years had 41% higher odds of having multiple recurrent visits compared to patients asged <18 years (OR 1·41, 95% CI 1·26–1·57), in contrast to 36% higher odds of having any recurrent visit (OR 1·36, 95% CI 1·27–1·45). However, some factors that were associated with the odds of having one or more recurrent visit were not significantly associated with the odds of having multiple recurrent visits. Household income, patient's disposition, and geographical location were not predictive of a patient's odds of having multiple recurrent visits, though these factors were significantly associated with odds of recurrent visits in general (Table 3).
Finally, although the logistic regression shows no significant difference in odds of recurrent visits between years, a proportional trend analysis showed a very slight but significant decreasing trend in multiple recurrent visits over time (slope = −0·0007, χ 2 trend = 3·94, P = 0·047).
DISCUSSION
To our knowledge, this study is the first to evaluate rates and sociodemographic and clinical predictors of visits for recurrent SSTI in the ED at such a large scale. We found that recurrent visits are a common problem, as over 16% of all SSTI patients returned to the ED at least one time for a similar condition in the 6 months following an initial SSTI. While these rates are significantly lower than the 28–39% recurrence rate of prior studies [Reference Miller15, Reference May16], prior studies included only patients with purulent wounds that could be cultured. However, as discussed below, our study examines only those patients that presented to the ED that subsequently sought care for their recurrent SSTI in the ED. Thus our estimates may be lower than true recurrence rates as some patients may have sought care for recurrent infections in non-ED settings such as clinics or with their primary-care provider.
SSTIs are disproportionately seen in EDs. In this large sample, we found multiple sociodemographic factors associated with recurrent ED visits for SSTIs, including age, race/ethnicity, and insurance status. The most surprising of these was race, as previous studies have not identified race-associated differences in recurrence rate [Reference Miller15, Reference May26]. However, it is likely that the observed difference is related to differences in healthcare utilization patterns, and not differences in predisposition to recurrent SSTIs. Disparities in healthcare utilization and outcomes could potentially be addressed with ED-based interventions to improve appropriate treatment to decrease risk recurrence, such as education on home-based management and hygiene, as well as linkages to appropriate care.
Interestingly, while patients seeking care in the ED generally are more likely to be underinsured and of minority status, we found that minority patients and those with Medicaid status were less likely to have a recurrent SSTI visit, whereas lower household income was associated with increased likelihood of a recurrent visit. This conflicting result may perhaps be explained by the higher rate of recurrent visits among self-pay patients (typically a poorer group). Together these findings suggest that having Medicaid may provide access to additional resources (e.g. primary-care providers) compared to those with no insurance that prevents ED recidivism. Further investigation of socioeconomics and SSTI recurrence is warranted.
We found that certain comorbidities, specifically obesity, drug or alcohol abuse, and liver disease were associated with increased likelihood of recurrent SSTI visits. While CA-MRSA, a primary cause of SSTIs [Reference Frazee1–Reference Zetola4], can strike healthy individuals, common comorbidities may be important predictors of community-acquired S. aureus infection [Reference David and Daum11, Reference Thana27]. Our findings suggest these populations are not only at higher risk of SSTIs but of SSTI recurrences as well.
Recent data on SSTIs in more than 2 million people found that diabetics have a fivefold increased SSTI incidence (4·9% vs. 0·8% per year) as well as higher rates of SSTI complications, including hospitalization, bacteraemia, and sepsis [Reference Suaya28]. Recent data also suggest obesity is a risk factor for CA-MRSA, including recurrent invasive disease [Reference Thana27]. Interestingly, we found that diabetes was associated with a lower risk of recurrent SSTIs, as were AIDS and cancer. The reasons for this are unclear, but may stem from the fact that these populations have lower barriers to access care and perhaps present in an earlier stage of infection that is less likely to relapse or are perhaps treated more aggressively than non-diabetic patients. Hospitalized patients had decreased odds of recurrent visits, which may reflect a more thorough evaluation and aggressive medical and surgical treatment before being released or perhaps access to post-discharge care that patients who are not hospitalized lack (see Supplementary Tables S2 and S3).
Most studies of MRSA-related SSTIs have been performed in urban areas, where the epidemic was first described [Reference Moran3]. Data on the incidence of SSTIs or CA-MRSA in non-urban or suburban regions other than Native American communities are scarce [Reference David29, Reference Stemper, Shukla and Reed30]. In this study, patients in low-density or rural areas of California had significantly reduced odds of recurrent visits. Reasons for this are unclear and perhaps related to rural areas sought care for their infections with primary-care providers. Alternately, prior studies have demonstrated that household crowding is associated with CA-MRSA infection and recurrence [Reference Golding31] and it is possible that persons living in areas of low density may experience less household crowding, although they face increased barriers to access which may reduce their recurrent visit rate [Reference Casey, Thiede Call and Klingner32].
Hospitalization for patients diagnosed with cutaneous abscess in the ED is uncommon, with fewer than 5% requiring admission [Reference May26]. In terms of treatment, while we lacked much clinical detail, patients with drained or aspirated SSTIs had higher odds of a recurrent visit compared to patients who did not receive these treatments. Although we were unable to confirm a specific diagnosis of cellulitis versus abscess in these patients due to coding vagaries (see below), the higher odds of recurrent visits associated with these procedures may reflect that cutaneous abscess, which are associated with CA-MRSA [Reference Taira6, Reference Pallin7], have higher rates of recurrence compared to non-suppurative cellulitis.
As noted above, one major limitation of our investigation is that the databases that we used allowed us only to examine SSTI recurrences that represent to EDs. Thus we cannot truly examine recurrence rates in this population and instead are focused on ED recidivism, which likely significantly underestimates recurrent disease. Additionally, our design may introduce bias in our analysis as some subpopulations may be more likely to receive care outside EDs for recurrent infection. Nevertheless, EDs remain a primary source of care for a large number of patients with SSTIs [Reference Taira6, Reference Pallin7] and it may be unlikely that a single ED visit for an SSTI would change a patients access or preferred point of care for a semi-urgent issue. Another limitation is our use of an administrative datasets to try to categorize of SSTI subtypes; more specifically, the ICD-9 billing code for abscess and cellulitis is the same (682.XX) and administrative databases cannot untangle these two forms of SSTI and these infection subtypes likely have different aetiologies and prognoses [Reference Stevens33]. Cutaneous abscesses may be more likely to recur than other types of SSTIs, possibly due to their association with CA-MRSA [Reference May16] the most common bacterial cause for culturable SSTIs nationwide [Reference Frazee1–Reference Zetola4]. While we were not able to assess MRSA as an aetiology of SSTI due to the lack of microbiology results in these datasets, our assumption that cutaneous abscesses are driving the higher recurrence rate is supported by the higher odds of a recurrent ED visit in adults aged 15–44 years, since CA-MRSA abscesses are more likely to occur in this population [Reference Moran3]. However, secular S. aureus epidemiological trends, which may have peaked in the mid-2000s and subsequently decreased nationwide, may also be driving some of the results [Reference Moran34, Reference Delorenze35].
There are additional limitations to our study. Our analysis did not account for patients that moved out of the state, came from out of the state, or died. Thus, some of the associations identified in this analysis may be more indicative of patients who are likely to seek follow-up care at an ED rather than those who are likely to have recurrent SSTIs. Additionally, reliance on ICD-9 codes may not have captured all patients presenting to the ED for an SSTI, as we only included patients with a primary diagnosis that met our criteria. The finding that admitted patients were significantly less likely to have a recurrent visit for SSTI leads to concerns that more critically ill patients with sepsis as their primary diagnosis may have been excluded.
Finally, SEDD is an administrative dataset and information on potentially important clinical variables, behavioral variables, and treatment is limited or non-existent. Given literature that suggests antibiotic treatment may be associated with decreased recurrent lesions and improved initial outcomes [Reference Talan36, Reference Talan37], we were unable to evaluate the role of antibiotic treatment in recurrent visits. The lower threshold for antibiotic treatment in immunosuppressed patients is also likely an important confounder. Additionally combining various categories of SSTIs (e.g. cellulitis, abscess, furunculosis), may be conceptually limiting in our analysis. Nevertheless, while we were unable to verify the diagnosis of abscess, the presence of aspiration or incision and drainage procedures may serve as a proxy for cutaneous abscess. We were also unable to confirm that subsequent ED visits for SSTIs were not associated with treatment failure for the initial SSTI rather than a true recurrence, especially given patients will often return to the ED for re-evaluation per standard ED practice. However, the exclusion of visits within 2 weeks of the initial visit likely diminishes this likelihood.
In conclusion, we found multiple sociodemographic factors and comorbidities associated with returning to the ED for a recurrent SSTI in a large population of patients in California. Identification of patients at risk for recurrent ED visits for SSTIs could help target therapy such as improving care access, or perhaps treating high risk patients with more appropriate antibiotics. Further investigation should better define the characteristics of populations at risk for recurrent SSTI visits, including their infection subtype, as those at risk for recurrent infection could be targets for interventions that could reduce the burden of repeat visits for this very common condition.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816002855.
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.






