INTRODUCTION
In wildlife disease epidemiology, a spillover host can be defined as one that acquires infection but cannot independently maintain the disease cycle at a population level; in contrast, ‘spillback’ involves the back transmission of infection from such a spillover host to a host capable of independent pathogen maintenance, sufficient to re-establish a self-sustaining disease focus at the population level. The consequences of spillback infection from a spillover to a maintenance host have variable impact for the persistence of disease in a multi-host system [Reference Kelly1, Reference Nugent2]. For example, brucellosis and bovine tuberculosis (TB) were probably introduced to North America with cattle in the early 1900s and were transmitted or ‘spilled-over’ to wild cervid populations. Today, back transmission from elk and deer is thought to be the main source of recurrent spillback infection for domestic cattle and thwarts attempts to eradicate those diseases [Reference Conner3, Reference Rhyan and Spraker4]. Spillback transmission is usually inconsequential in a multi-host disease system where the disease is long-established and unmanaged in the primary maintenance host, because the magnitude of inter-species transmission is negligible compared with intra-species transmission. However, spillback may be disproportionately important where efforts are being, or have been, made to eradicate infection from the maintenance host. We considered the latter situation here and used simulation modelling to explore the effects of some different management strategies on the risk of spillback and overall disease persistence within a disease system with more than one wildlife host.
Bovine TB is recurrent in farmed cattle and deer in New Zealand [Reference Ryan5], where attempts to manage the disease have been complicated by its maintenance in a prevalent wildlife species, the introduced Australian brushtail possum (Trichosurus vulpecula). On-going efforts at TB mitigation are focused on a combined approach of herd surveillance testing (and culling of infected livestock), livestock movement control, and possum population control, the latter since localized control of possums has been demonstrated to reduce herd reactor rates and to lower the incidence of Mycobacterium bovis infection in adjacent livestock [Reference Caley6, Reference Kean, Barlow and Hickling7]. New Zealand is now on course for declaration of official TB-free status when its national infected herd period prevalence rates remain below 0·2% for three consecutive years, under criteria set by OIE guidelines [Reference Knowles, Hunter, Rush, Nolte and Fagerstone8], with future efforts aimed at regional TB eradication [Reference Hutchings, Hancox and Livingstone9]. However, in addition to possums, several other introduced mammalian species are present among New Zealand wildlife, and some are infected with M. bovis frequently enough [Reference Coleman and Cooke10] that the risk of spillback transmission of infection from these hosts is of concern, particularly if it results in re-establishment of TB in the possum population. The presence of multiple sympatric wildlife hosts in New Zealand could impede efforts at TB eradication, therefore the Animal Health Board (AHB; the national agency responsible for TB control in New Zealand) has targeted two regional areas where TB is long-established in a multi-host wildlife complex (Hokonui Hills and Hauhungaroa Range), to determine whether TB eradication is feasible under such conditions [Reference Hutchings, Hancox and Livingstone9, 11].
The Hauhungaroa Range is in the central North Island (38°44′S, 175°35′E) and is the subject of this study. It is a low mountain range, with a central core of native forest (largely reserved for conservation) surrounded by pastoral (sheep, cattle, deer) farmland and some exotic plantation forest. The native forest can support high numbers of possums (estimated carrying capacity of 300–700 possums/km2), moderate densities of wild red deer (Cervus elaphus scoticus, estimated density of 6 deer/km2 in 1994 [Reference Nugent, Fraser and Coleman12]), and a lower but highly variable number of wild pigs (Sus scrofa [Reference Nugent, Fraser and Coleman12]). TB due to M. bovis has long been present in possums, deer and pigs in the Hauhungaroa Range [Reference Pfeiffer13–Reference Nugent15]. Since the early 1990s, large parts of the area have been subject to intensive possum control through poisoning, although full control coverage was probably not achieved until 2005, the same year in which the last confirmed cases of TB were identified in possums from the area [Reference de Lisle, Yates and Coleman16]. Since then, and as late as 2010, there have been credible reports of deer bearing tuberculous lesions still being killed in the area, including by professional hunters experienced in the field recognition of TB (D. Wilson, Hunt South Ltd, personal communication).
One area of concern for disease managers is whether this apparent ‘tail’ of residual TB in red deer poses a significant risk to the goal of local disease eradication. Wild deer are not normally considered to be TB maintenance hosts in New Zealand, because population densities are insufficient for independent maintenance of the TB cycle [Reference Nugent2], evidenced by large-scale field trials which show that TB prevalence in deer declines to near zero levels when possums, but not deer, are controlled [Reference Nugent15]. However, individual animals, particularly females, can live for up to 15 years [Reference Weigl and Jones17], so theoretically they could carry infection for long periods of time before becoming infectious close to, or after, death [Reference Nugent15, Reference Williams18]. Such infected deer could re-establish TB in possums long after it has been eradicated from the latter population, particularly if possum control ceases and possum densities increase again to exceed the moderately low threshold nominally required for TB persistence [Reference Barlow19]. To reduce this risk, the current management strategy is to maintain possum numbers below that threshold density for at least 15 years, based on the premise that any infected deer present at the start of possum control should have died by then. For large areas of native forest in which deer densities are high enough to be of concern, such as the Hauhungaroa Range, possum control is likely to involve 3–4 aerial poisoning operations at about 5-year intervals to fulfil the eradication aim [20]. That duration and intensity of possum control is likely to be substantially more than is needed to eliminate TB from possums alone, so there is a major opportunity cost in addressing the risk of potential spillback transmission of M. bovis infection to possums.
In this paper, we present findings from a simulation model used to assess the duration of the risk period of M. bovis infection being transmitted back to possums from deer and re-establishing in possums, once TB is eradicated from possums, using the Hauhungaroa Range as a case study. The model predicted a substantial spillback risk period, so we then explored whether concurrent control of the TB-affected deer population would reduce the duration of the risk period sufficiently and cost-effectively enough to be warranted as an additional tactic in the TB eradication strategy.
METHODS
Overall modelling approach
The interactions of the two species of interest in this study (deer and possums) were explored, while acknowledging that other wildlife hosts susceptible to M. bovis infection are also present in New Zealand [Reference Coleman and Cooke10] and could, feasibly, impact on the dynamics of this two-species model. In principle, this two-host species model for TB requires four potential routes of transmission to be evaluated in full: deer to deer, possum to possums, possum to deer and deer to possum. However, intra-species transmission in deer is considered to occur only rarely in the low to moderate density wild deer populations in New Zealand, with most M. bovis infection in deer attributed to transmission from sympatric tuberculous possums [Reference Lugton14, Reference Nugent15]. Deer-to-deer transmission was therefore not modelled.
Possum population and TB dynamics (possum to possum transmission), and the effect of possum control via 1080 poisoning [21] on these dynamics, were modelled using the non-spatial TB-possum model developed by Barlow [Reference Barlow19]. Deer population dynamics were modelled using an age- and sex-structured model, described below, where possum-to-deer transmission was modelled as a function of possum density. Spillback transmission of M. bovis infection from deer to possums was the process of primary interest in this study, and was assumed to occur via possums investigating or scavenging tuberculous deer carcasses. Nugent [Reference Nugent15] has reported that possums will readily investigate and make physical contact with animal carcasses: of 19 deer carcasses experimentally placed in New Zealand field conditions and monitored by night vision cameras over periods of 2–5 weeks, 17 carcasses were investigated and sometimes fed upon by at least one possum (with a total of 202 contact events recorded). In the present study, we hence assumed that the probability of spillback occurring was mainly a function of the number of tuberculous deer carcasses in the environment. A spatial possum-TB model [Reference Ramsey and Efford22] was then used to independently derive a relationship between possum density and the probability that a single possum, infected via contact with a tuberculous deer carcass, could re-establish a self-sustaining TB focus in the possum population (i.e. a true spillback event).
For the purposes of this study, we based calculations on the assumption that both TB and the prevalence of TB are represented by clinical disease in both possums and deer, involving archetypal macroscopic necrotic lesions of the type found in field cases of disease [Reference Coleman and Cooke10, Reference Lugton14, Reference Nugent15] (rather than by subclinical infection with M. bovis [Reference de Lisle, Yates and Coleman16]) because we consider this represents the disease phase most relevant to intra- and inter-species transmission.
The parameter values used in the model simulations represent a range of reliability. The parameters describing the possum population and TB dynamics are the most well established, followed by the deer population parameters, with the inter-species transmission parameters the least well-studied and the most uncertain. Accordingly we assessed the sensitivity of the model predictions to the most critical parameter for estimating spillback risk, the probability of deer-to-possum transmission given a possum encounter with a deer carcass.
Modelling TB in possum populations and possum control
Trends in possum density, and in the prevalence of TB in possums, both with and without possum control, were modelled using a discrete time derivation of Barlow's non-spatial possum-TB model [Reference Barlow19]. We used the same parameter values as Barlow, except the disease transmission coefficient and the disease aggregation parameters were changed to β = 1·2 and κ = 0·03, respectively, to produce a 2·5% TB prevalence in possum populations at the assumed equilibrium density of about 650 possums/km2. This approximates the TB prevelances recorded around the periphery of the Hauhungaroa Range in 1982–1983 (2·5% [Reference Pfeiffer13]), and in the southeastern part of the area in 1994 (2·2% [Reference Fraser, Coleman and Nugent23]), before possum control began there.
Modelling TB in deer populations and deer control
An age- and sex-structured TB model of the deer populations was developed, with two infection groups (TB−, TB+), two sex groups (females, males) and 16 age groups (years 0–15). Deer density and TB levels were modelled in discrete time using an annual time step. Both fecundity and survival were assumed to be density dependent (see Supplementary Table S1 in the online Appendix) as has been observed in red deer populations overseas [Reference Guinness, Clutton-Brock and Albon24, Reference Coulson25]. We assumed no twinning, an equal sex ratio at birth, and proportions of females producing offspring per annum that were low for yearlings, high for animals aged 2–9 years, and declined thereafter. The proportion of females producing offspring per annum was the maximum productivity multiplied by a Ricker-type density-dependent function [Reference Ricker26]. Similarly, proportional survival for each sex/age group was the maximum possible survival modified by Ricker-type density dependence. Survival was assumed to be relatively low in fawns and yearlings, very high for animals aged 2–5 years then declined with age for animals aged 6–15 years. Stags in the 0–2 years age groups were assumed to have lower survival relative to hinds, producing an adult sex ratio biased towards females similar to that observed in unhunted populations [Reference Nugent, Fraser and King27]. This combination of vital rates produced a maximum instantaneous rate of increase of r m = 0·29 from very low deer densities, which is similar to maximum population rates of increase measured in overseas studies (Table 1 in Forsyth et al. [Reference Forsyth28]).
Table 1. Proportional kills of deer population under different deer control regimens with deer sex and age
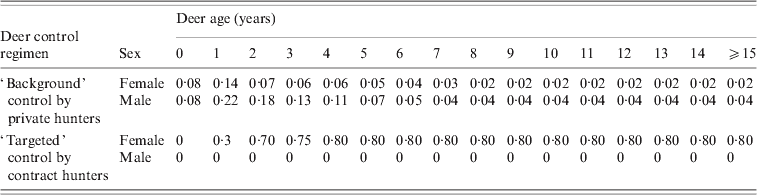
The proportion of deer infected per annum was modelled as an exponential function of the tuberculous possum density and the instantaneous incidence rates listed in the online Appendix. Incidence rates were assumed to be highest for deer aged 1–4 years and higher in stags than hinds, as suggested by the age- and sex-specific TB prevalence recorded in deer from the Hauhungaroa Range during the early 1990s [Reference Nugent15]. Mortality of deer due to TB is apparently rare [Reference Williams18], and was therefore assumed to be minimal. We also assumed that 12% of infected deer resolved the disease per annum, as estimated by Nugent [Reference Nugent15].
The deer population of the Hauhungaroa Range has been subject to several decades of largely unrestricted year-round private (recreational and commercial) hunting [Reference Nugent, Fraser and Coleman12]. While we assumed that this would continue (see Table 1), previous modelling [Reference Nugent and Choquenot29] suggested it would be uneconomical to try to achieve increased deer control via some form of enhanced recreational hunting. Instead we simulated two other deer control options available in New Zealand, namely targeted poisoning and use of contract professional hunters. Poison baiting of preferred foliage has occasionally been trialled as a deer control tool in New Zealand, with a prior field trial in the Hauhungaroa Range indicating high (>80%) percentage kills of red deer [Reference Sweetapple30]. We assumed that poisoning was unselective with respect to age and sex, as its application to foliage makes it accessible to all deer. The second deer control approach modelled was professional ground hunting. This option enabled simulation of scenarios involving targeting of defined age-sex groups within the deer population, specifically adult female deer, the age-sex group considered most likely to carry TB for the longest periods of time.
Equations and parameter values describing the deer population TB model are provided in the online Appendix.
Modelling deer-to-possum back transmission of M. bovis and estimating the spillback risk
Back transmission
Although there is empirical evidence of TB transmission from deer to possums in the field [Reference de Lisle31, Reference Mackereth32], the mechanism and rate at which this happens is unknown. For this paper, the assumed mechanism of back transmission was by possums contacting tuberculous deer carcasses [Reference Nugent15, Reference Ragg, Mackintosh and Moller33, Reference Barron34]. Nugent [Reference Nugent2] suggested the chance of deer-to-possum transmission is highest when hunters decapitate tuberculous deer and leave the head (but little else) at the kill site, thereby increasing the likelihood that possums would come into contact with the retropharyngeal lymph nodes, the most common site of gross lesions in tuberculous red deer [Reference Mackintosh35]. Under conditions of experimental infection, tuberculous lymph nodes of red deer have been shown to contain in excess of 105M. bovis bacilli/g of affected tissue [Reference Griffin36], representing a readily available source of infectious material; possums are known to be highly susceptible to M. bovis infection, and exposure to as few as 10 bacilli has been shown to establish generalized TB in experimentally challenged animals [Reference Aldwell37]. Accordingly, we assumed a relatively high infection rate of b=0·25 (i.e. one in four possums encountering a tuberculous deer carcass becomes infected). However, we also investigated how the simulated number of back-transmission events changed over a range of values of b, i.e. assuming higher and lower deer-to-possum re-infection rates.
We assumed that the probability of an individual possum acquiring TB from a deer carcass was:
where α is the possum encounter rate of deer carcasses, C is the density of tuberculous deer carcasses in the environment, and b is the probability that a possum becomes infected given an encounter with a tuberculous deer carcass. The rate at which an individual possum is likely to encounter a deer carcass was approximated by dividing the area of a typical possum home range [Reference Cowan, Clout and Montague38] by that of a deer's home range [Reference Nugent2], i.e. α = 0·02 km2/2·5 km2=0·01. The density of tuberculous deer carcasses was estimated by tallying within the model the number of M. bovis-infected deer that died each year.
Spillback risk
Once a single possum has become infected with M. bovis through contact with a tuberculous deer carcass, there is some probability that it will subsequently infect other possums and re-establish a disease focus within the possum population (true spillback). This risk was estimated for the Hauhungaroa Range case study using the individually based spatially explicit possum TB spatial model of Ramsey & Efford [Reference Ramsey and Efford22]. Possum population dynamics were driven by possum-carrying capacity, which was derived at a pixel resolution of 50 m following Warburton et al. [Reference Warburton, Cowan and Shepherd39], and which averaged 6·7 possum/ha. Possum population density within the simulated area was initialized at 10%, 20%, …, 100% of carrying capacity. We assumed the spatial TB transmission coefficient (β’) was 0·32, as this produced an observed disease prevalence of 2·5% when the possum population was at equilibrium.
In year 1 of each simulation, a single tuberculous possum was placed randomly within the simulated population and the model was run for 5 years to assess whether TB persisted in the population. The presence of tuberculous possums after 5 years was assumed to indicate TB establishment, as there is a <1% probability that the original infected individual would survive for ⩾5 years (given an assumed annual instantaneous mortality rate of 1·1 [Reference Ramsey and Efford22]).
For each initial relative density the model was run 200 times, and the proportion of simulations with TB still present in the population 5 years after the occurrence of the initial back-transmission event was used as the probability of a single re-infected possum re-establishing TB in the Hauhungaroa possum population. This produced the expected positive relationship between relative possum density and the probability of TB establishment (Fig. 1).

Fig. 1. Probability of a single tuberculous possum re-establishing tuberculosis (TB) in the possum population over a range of relative possum densities (N/K p). For these simulation runs (n = 200) possum carrying capacity (K p) averaged 6·7 possums/ha.
Simulation of deer and possum control scenarios
Five deer and possum control scenarios were considered and modelled:
(1) No possum control plus annual ‘background’ control of deer by private hunting as per Table 1. This represents the regimen that prevailed in the Hauhungaroa Range before intensive possum control began in 1994.
(2) As per scenario 1, but with possum control (by aerial poisoning) at a 5-year frequency with an initial control efficacy of 95% (and, in addition, a 30% by-kill of deer); and thereafter an efficacy of 85% with no associated deer by-kill (simulating the use of baits coated with a proprietary deer-repellent [Reference Speedy40] that has been reported to restrict by-kill of non-target cervids to <5% of pre-control population levels).
(3) Possum control with background control of deer as in scenario 2, plus a one-off foliage-baiting operation to provide an 80% kill of deer (based on Sweetapple [Reference Sweetapple30]) in the year following initial possum control.
(4) Possum control with background control of deer as in scenario 2 plus annual ‘targeted’ deer control (culling of adult females as per Table 1) over 5 years following the initial possum control.
(5) Possum control with background control of deer as in scenario 2 plus annual ‘targeted’ deer control (culling of adult females only as per Table 1) over 5 years following the eradication of TB from possums.
The simulations were run for 20 years with the initial possum control operation occurring in year 1 and background deer control occurring every year. The 5-year frequency of aerial possum control operations was modelled using poison deployed at years 1, 6, 11. All controls were implemented as a simple proportional removal from the population. The estimate of a 30% by-kill of deer during initial possum control is based on data from the first aerial 1080 poisoning of parts of the Hauhungaroa Range in 1994 [Reference Coleman, Fraser and Nugent41]. We assumed private hunters left potentially infectious carcasses (or parts thereof) at kill sites, but that the professional contract hunters did not.
To account for demographic stochasticity, 5000 replicate simulations were run for each combination of control scenario (scenarios 1–5) and probability of deer-to-possum infection (b = 0·1, 0·25, 0·5, 0·75). The outcomes calculated from these simulations were the mean time until TB was eradicated from both possums and deer, the cost of the possum and deer control operations conducted, and the probability of back transmission and disease re-establishment occurring after TB had been eradicated from the possum population.
Cost–benefit analysis in simulations
Cost–benefit ratios of deer control were assessed relative to scenario 2, which represents the current operational practice of three possum control operations and no additional deer control. Cost–benefit ratios were expressed as (a) the reduction in eradication time in years for each NZ$/km2 spent on deer control and (b) the proportional reduction in back transmission and re-establishment risk for each NZ$/km2 spent on deer control. Adjusted for inflation, the foliage-baiting trial conducted in the Hauhungaroa Range [Reference Sweetapple30] was estimated to cost NZ$ 1500/km2 at 2012 prices. The cost per km2 of possum control was assumed to be NZ$ 3600 for an initial 95% control, representing aerial broadcast delivery of baited 1080 poison with a single non-toxic pre-feed as typical operational practice [Reference Nugent42]. Subsequent control operations for possums were assumed to cost NZ$ 2000/km2, conservatively representing less intensive aerial control (according to that recently recommended by reduced sowing rates as a result of aggregated bait delivery, rather than broadcasting [Reference Nugent42]). Targeted deer control by contract hunters was estimated to cost NZ$ 700/km2 [Reference Sweetapple30], while deer control by private (recreational) hunting was assumed to incur no cost to TB managers.
RESULTS
With no possum control and with just background deer control (scenario 1), equilibrium deer densities were 11·4 deer/km2 with a TB prevalence of 33%, while equilibrium possum densities were 652 possums/km2 with a TB prevalence of 2·5% (Figs 2 and 3). The deer sex ratio was 61 males for every 100 females. TB prevalence was highest in male deer aged 4–5 years, at around 58%, and in females aged 5–6 years, at around 50%, declining with age thereafter for both sexes (Fig. 2). These predictions are broadly consistent with empirical evidence from the Hauhungaroa Range before intensive possum control was initiated in 1994 [Reference Nugent15].

Fig. 2. Proportion of deer population in different age, sex and tuberculosis (TB) infection groups when the possum population is uncontrolled and deer populations are subject to background private hunting (scenario 1). Length of bar for each age, sex and TB status group represents a proportion of the total population.
Under standard possum control and background deer control (scenario 2), the model predicted TB eradication from the possum population after 7 years (Table 2, Fig. 3) and from the deer population after 14·1 years, at a total cost of NZ$ 7600 km−2. Deer densities were reduced following possum control, averaging 10·3 km−2 due to by-kill from the initial possum poisoning operation (Table 2, Fig. 3). When we ran the model with differing strategies for deer control (control scenarios 3–5), the time to eradicate TB from deer was reduced as expected but the incremental gains for this additional control were small, with the best being obtained from control scenario 3 which predicted TB eradication from deer after 12·3 years (Table 2, Fig. 3).

Fig. 3. Changes in mean deer and possum density, and tuberculosis (TB) prevalence predicted under control scenarios 1–5. Black lines indicate host population density, Grey lines indicate TB prevalence within host population. Arrows along the horizontal axes refer to major pest population reductions of ⩾80% due to targeted poison control operations (black symbols), or 30% due to by-kill (grey symbols), for scenarios 2–5.
Table 2. Results from the deer–possum–TB simulation model under five control scenarios. Results are means of 5000 replicate simulations, run for 20 years with possum population control in years 1, 6 and 11

n.a., Not applicable.
* Assessed over years since initial possum control.
† Total cost of three aerial possum control operations = NZ$ 7600/km2.
‡ Number of events from time TB eradicated from possums to time TB eradicated from deer.
§ Assessed over 20 years of simulation.
Under scenario 2 (standard possum control and background deer control), the probability of M. bovis back transmission from deer to possums sufficient to re-establish disease in the possum population at least once after TB was originally eradicated from the possum population varied from 0·01 to 0·11, depending on the parameter value of b (Table 3). Deer control reduced that risk by 37–75%, with scenario 3 providing the greatest reduction.
Table 3. The estimated cost–benefit ratios of deer control under scenarios 3–5 relative to possum control alone (scenario 2) and the sensitivity of model simulations to the probability of a possum becoming infected with M. bovis given an encounter with a tuberculosis (TB) deer carcass

* Re-establishment of TB in the possum population, i.e. a true population-level spillback event.
Foliage baiting (control scenario 3) was the most cost-effective method out of the three deer control options for reducing both the time until TB eradication and the spillback risk. However, the absolute reduction in the spillback risk period was <2 years (Table 3), and accordingly there was little cost saving through avoiding a further repeat of aerial poisoning of possums.
DISCUSSION
Model predictions
Overall, TB was predicted to persist in deer for about 14 years after possum control began. Consistent with that, TB prevalence in deer in the eastern side of the Hauhungaroa Range is reported to have declined from ∼30% in 1993 to near zero in 2003 after control operations starting in 1994 had reduced possum densities to low levels [Reference Nugent15]. However, possum numbers were not reduced uniformly throughout the Hauhungaroa Range until 2005 [Reference Coleman, Fraser and Nugent43], and hence the modelling results suggest that some risk of spillback transmission of infection (albeit small) could persist in the Hauhungaroa Range until about 2020, with the greatest risk being in the western central part of the Range, the last area where possums were controlled successfully [Reference Nugent44].
In New Zealand, empirical evidence suggests that lethal control of maintenance host (possum) populations alone is sufficient to eventually eliminate TB from secondary spillover wildlife hosts such as ferrets [Reference Caley and Hone45], pigs [Reference Nugent44] and deer [Reference Nugent15]. The difference among these three secondary spillover hosts, and the cause of the concern addressed here, is their longevity. The intrinsic exponential rate of increase in possum populations is moderate (∼0·35–0·45; see review by Nugent et al. [Reference Nugent46]) so possum populations can recover to moderate or high densities within 5–10 years of intensive control. Both modelling and empirical evidence suggest the threshold density for TB persistence in possums is low [Reference Nugent15, Reference Barlow19, Reference Ramsey and Efford22], and this threshold could theoretically be exceeded in as little as 5 years after the cessation of control. Feral ferrets and pigs rarely live longer than that [Reference Jedrzejewski47, Reference Clapperton, Byrom and King48], but wild deer, particularly females, do [Reference Weigl and Jones17]. Thus the longevity of deer determines the duration of the spillback risk period which, under the deer mortality rates assumed here, was about 7 years post-TB eradication from possums.
Increasing the deer mortality rates by way of deer control did not greatly reduce the duration of the spillback risk period, at least for the control scenarios modelled here. Even a one-off 80% reduction in deer numbers (by targeted foliage-bait poisoning; scenario 3) produced only a decrease in the risk period of <2 years. Similarly, moderately intensive control of the adult female deer population (scenarios 4 and 5) also resulted in only small predicted reductions in the spillback risk period, despite females making up 60% of the deer population overall and having double the number of tuberculous deer in the older (>10 years) age groups. This is because we applied a strict definition criterion of TB eradication in our interpretation of results: we considered that the presence of just one tuberculous deer in, for example, a total population of 720 (the average under scenario 3) would indicate that TB was persistent and represented a positive spillback risk even though that TB prevalence was actually very low (0·1%) and the spillback risk was accordingly extremely small.
Under the default parameter values, and with no additional deer control, the predicted probability of TB spillback with re-establishment occurring in possum populations in the Hauhungaroa Range was about 6% (0·0566, 95% confidence interval 0·0504–0·0634). However, we acknowledge that this result is sensitive to the parameter value estimates, some of which are poorly known. In particular, the predictions were highly sensitive to the value of b, the rate of possum infection per encounter with a TB deer carcass. As already noted, that parameter has never been measured, but there is suggestive evidence that spillback transmission from deer to possums occurs in New Zealand. For example, a TB outbreak that occurred in the early 1990s at Waipawa (Hawkes Bay region, North Island) was of a strain of M. bovis not previously recorded in either livestock or wildlife in the vicinity [Reference Mackereth32]: the strain appeared for the first time in possums soon after deer had been relocated there from the Otago region (South Island), a distance of some 1000 km (and the Otago deer were subsequently found to be carrying that particular strain). More anecdotally, it is known that possums readily investigate deer carcasses and make physical contact with exposed internal tissues [Reference Nugent15]. In an unpublished study, Nugent & Whitford [Reference Nugent and Whitford49] examined this phenomenon further, by depositing 39 deer carcass remnants containing individual lymph nodes injected with a marker dye at each of three different field sites; dye was found in 7% of possums at each of two sites (and none at the third), confirming that some possums do indeed make contact with high-risk tissue in a deer carcass, in a manner plausible for the acquisition of M. bovis infection. Taking into account both the observed ability of possums to make contact with lymphoid tissues in deer carcasses [Reference Nugent and Whitford49] and the demonstrated survival of M. bovis bacilli in animal carcasses [Reference Barron34], our model predictions for spillback risk selected a conservatively high default value of b=0·25, or 1/4 cases of a possum encountering a tuberculous deer carcass resulting in the possum acquiring M. bovis infection. At this rate of infection, the risk of spillback occurring was reduced by 41–73% by additional control of deer, due to the removal of some of the source infection (i.e. tuberculous deer) from the population. While this represents a good proportional reduction in risk, the low baseline risk of spillback, combined with the high cost of hastening a decline that would occur anyway, probably argues against additional expenditure on deer control.
Management implications
The current AHB strategy for possum control in large heavily forested areas is to maintain low possum densities for at least 15 years by applying high-intensity lethal control on at least three occasions each about 5 years apart [11]. This strategic approach and time-frame was designed and adopted with the risk of TB spillback from deer in mind, and our modelling quantitatively supports this approach. While TB eradication from modelled possum populations was usually achieved with just two aerial controls, a third aerial control provides the benefit of sustaining low densities of possums over the time of spillback risk. A third aerial possum control also provides some insurance against previous incomplete control coverage or poor percentage kills (although in the unlikely event of that occurring, early detection via post-operational residual possum monitoring, and subsequent follow-up control, would be likely to occur first). Cost–benefit analysis indicates there would be no major saving in time to eradication by applying deer control in addition to standard possum control.
Cost–benefit ratios may be even lower than those presented since the costs of deer control used in the estimation are likely to be conservative. We modelled control as a simple proportional kill/removal from that cohort. In reality, proportional kill rates would be expected to decline with population density, resulting in increased effort expended or money spent to remove an individual deer at low compared with high deer densities [Reference Nugent and Choquenot29], which means that control costs may – if anything – have been underestimated. Further, our estimates only tallied monetary costs associated with the control; because wild deer populations are viewed by hunters as a valuable recreational resource [Reference Fraser50], imposing intensive deer control (particularly by poisoning) could incur major social antipathy and possible interruption to poison control operations, the mitigation costs of which were not included in our modelling.
CONCLUSIONS
Overall, based on the modelling conducted here, we conclude that deer control to reduce the TB spillback risk in large forested areas of New Zealand is rarely likely to be warranted in its own right. TB is predicted to drop out of wild deer populations once inter-species transmission is curbed by effective long-term possum control; additional money spent on deer control will do little to hasten that decline. This scenario is similar to that experienced in the Northern Territories of Australia in the 1980s and 1990s: there, TB persisted at a high prevalence in wild pigs, feral cattle and water buffalo [Reference McInerney, Small and Caley51]; however, subsequent eradication of TB was achieved by population control of the ruminant species alone, without the need for additional control of pigs [Reference Turner52]. In the New Zealand case, rather than population control of deer as a means of supporting TB eradication, selective culling and necropsy of deer as TB sentinels [Reference Nugent53] could be useful to confirm that a decline in disease prevalence is occurring, and provide data for calculating the likelihood that TB has been eradicated from possums [Reference Hutchings, Hancox and Livingstone9]. Low-level deer control may therefore be warranted on surveillance grounds, with the reduction in spillback risk being an incidental benefit.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268812002683.
ACKNOWLEDGEMENTS
We thank Drs Dan Tompkins, Pen Holland and Phil Cowan (Landcare Research) for reviewing this manuscript and Christine Bezar (Landcare Research) for the editing. This work was supported by a research grant from the Animal Health Board of New Zealand (Wellington).
DECLARATION OF INTEREST
None.










