Introduction
Diabetes mellitus (DM) is one of the leading causes of morbidity and mortality globally. Approximately 10% of the US population has DM [1, Reference Korbel and Spencer2]. The association of DM and the increased risk for infections has long been established in the clinical community [Reference Dryden3, Reference Peleg4]. Among individuals with DM, pneumonia is the most common infection managed in the hospital and the third most common infection treated in the emergency department (ED) [Reference Korbel and Spencer2]. Several studies have described the association of DM and increased risk for pneumonia [Reference Torres5, Reference Casqueiro and Alves6]. Individuals with DM may have increased susceptibility to pneumonia based on various factors, including dysfunctional immunity related to the harmful effects of hyperglycaemia, risk of aspiration, impaired lung function and other co-existing comorbidities [Reference Dryden3, Reference Peleg4, Reference Avishai, Yeghiazaryan and Golubnitschaja7]. However, there are limited data on the relationship between DM and risk for pneumonia, particularly as it applies to different populations and health care settings [Reference Torres5, Reference Muller8]. The objectives of this study were to (1) compare the prevalence and healthcare utilisation patterns for pneumonia in individuals with and without DM, and (2) identify risk factors for pneumonia in those with DM.
Methods
Data source
We analysed data from the Medical Expenditure Panel Survey (MEPS), a programme of the Agency for Healthcare Research and Quality (AHRQ). MEPS is a set of a comprehensive survey that provides national estimates of healthcare use, expenditures, payments and insurance coverage. It collects information from individual households, medical providers and employers in the USA. Data are collected in an overlapping panel design, with respondents undergoing five rounds of interviews in a two-calendar year period.
There are three main components to MEPS: The Household Component (HC), Medical Provider Component and Insurance Component. The MEPS HC includes data from household respondents with additional information supplemented from the Medical Provider Component. The HC provides person-level data on the demographics, health conditions, health status, healthcare use and payment for a representative sample of the non-institutionalised civilian population. Each event in the file represents a unique household-reported medical event, and details such as date of visit, type of care and condition codes are provided. The HC data organise medical events into sub-components of healthcare settings; three of the sub-components used in this work were ‘Hospital Inpatient Stays’, ‘Emergency Room Visits’ and ‘Outpatient Department Visits’. In addition, we accessed data from the Diabetes Care Survey (DCS), one of the five HC supplemental paper questionnaires provided to respondents with a self-reported diagnosis of DM.
Study design and definitions
We performed a retrospective, cross-sectional analysis of pneumonia prevalence, risk factors and healthcare utilisation patterns of individuals with DM, without DM and with DM-complications in 2014. In the MEPS interviews, survey respondents' health conditions were coded using the Clinical Classification Software (CCS) codes. The three healthcare settings studied were hospital inpatient, ED and outpatient clinics. Demographic characteristics, comorbidities and variables for diabetes management were extracted using the MEPS HC. Adults aged 18 years and older were included in the study. Individuals with DM were identified as those with a CCS code 049 or 050. A pneumonia event was defined with a CCS code 122. Individuals with DM-complications were defined as DM with documented retinopathy or nephropathy. Expenditures in MEPS represent amounts actually paid for health care services, including those collected for hospital inpatient care, ambulatory care provided in offices or hospital outpatient facilities, and care provided in the ED reported in the HC of the survey. Total expenses for a pneumonia event are defined as the sum of direct payments by households, private insurance, Medicare, Medicaid and other sources to providers of the care.
Data and statistical analyses
Population-based pneumonia prevalence rates were calculated as the annual number of pneumonia events divided by the corresponding US non-institutionalised population. Population denominators were derived from the MEPS HC. The MEPS estimate US populations based on sampled persons in the target population (civilian non-institutionalised) for the entire year. Prevalence estimates were calculated to compare total pneumonia events and healthcare utilisations among the different healthcare settings between the subgroups. Multivariable logistic regression models were used to identify independent factors associated with pneumonia among individuals with diabetes. This model contained age, sex, comorbidities (e.g. chronic heart disease, emphysema, chronic bronchitis, cancer, tobacco use), presence of diabetes complications, factors related to diabetes care (vaccines, medications) and duration of the disease. Generalised linear models were used to adjust for demographic and clinical characteristics to estimate adjusted mean expenditures for pneumonia. To account for MEPS' complex study design, all analyses were adjusted using weights, clustering and stratification. SPSS 24.0® (IBM Crop, Armonk, NY, USA) was used for all statistical analyses. Statistical significance was calculated using a P-value of <0.001.
Results
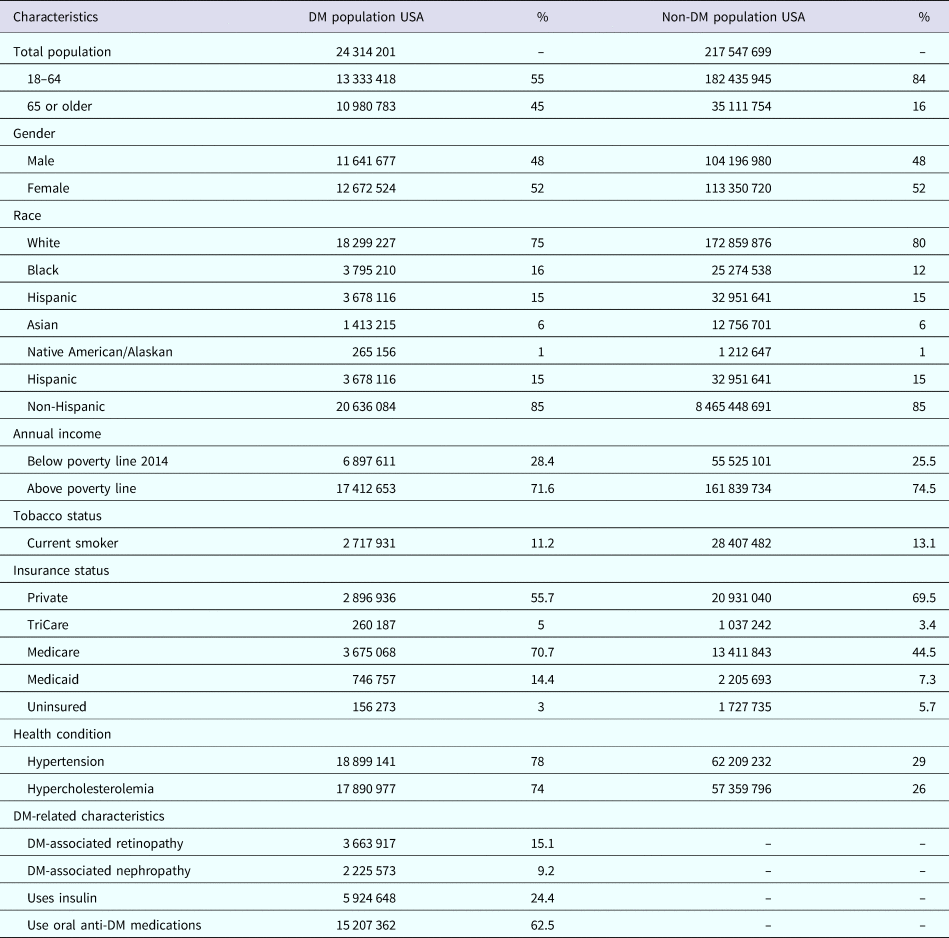
The data represented 24 million individuals with DM and 218 million without DM in the USA. A quarter of the individuals with DM had DM-complications. Table 1 compares the cohort characteristics. Overall, individuals with DM were older (66 vs. 52 years of age). Sex, race, educational level and annual income were distributed similarly between individuals with and without DM. There were a higher proportion of individuals with DM with comorbid health conditions compared to those without DM, including hypertension (78% vs. 29%) and hypercholesteraemia (74% vs. 26%). Among individuals with DM, the mean duration of DM diagnosis was 13.6 years. More than half of individuals with DM (62%) were on oral medication for DM management and a quarter (24%) were on insulin. Less than half of individuals with DM (49%) reported receiving an influenza vaccination during 2014 and 61% reported a history of receiving a pneumococcal vaccine.
Table 1. Baseline demographics
The overall population-based rate for a pneumonia event was 34 per 1000 persons for individuals with DM compared to 19 per 1000 persons for individuals without DM. Among individuals with DM, the population-based rate for pneumonia among those with DM-complications was twofold greater than those with DM without complications (60 per 1000 person vs. 28 per 1000 persons). Among individuals with DM, female sex (OR 2.1 (95% CI 2.08–2.10)), smokers (1.33, 1.30–1.36), presence of DM complications (1.15, 1.14–1.16) and history of pneumococcal vaccine (1.07, 1.07–1.08) were significant factors associated for a pneumonia event.
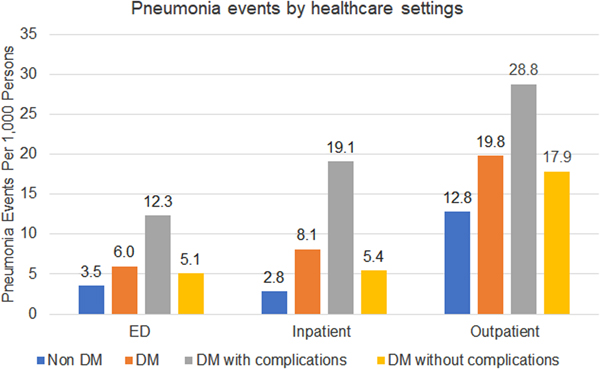
There were substantial differences in healthcare utilisation by setting among those with and without DM (Fig. 1). Compared to the non-DM group, individuals with DM were treated 1.8x, 2.6x and 1.4x more in the ED, hospital and outpatient, respectively. Among individuals with DM, those with DM-complications had higher healthcare utilisation across all healthcare settings: ED 1.8x, inpatient 2.6x and outpatient 2.8x. The most common pneumonia treatment setting overall was in outpatient clinics (65% of all pneumonia events). Among individuals with DM, inpatient treatment was used more frequently than ED (24% vs. 18%), while individuals without DM were treated similarly between ED and inpatient (17% vs. 15%).
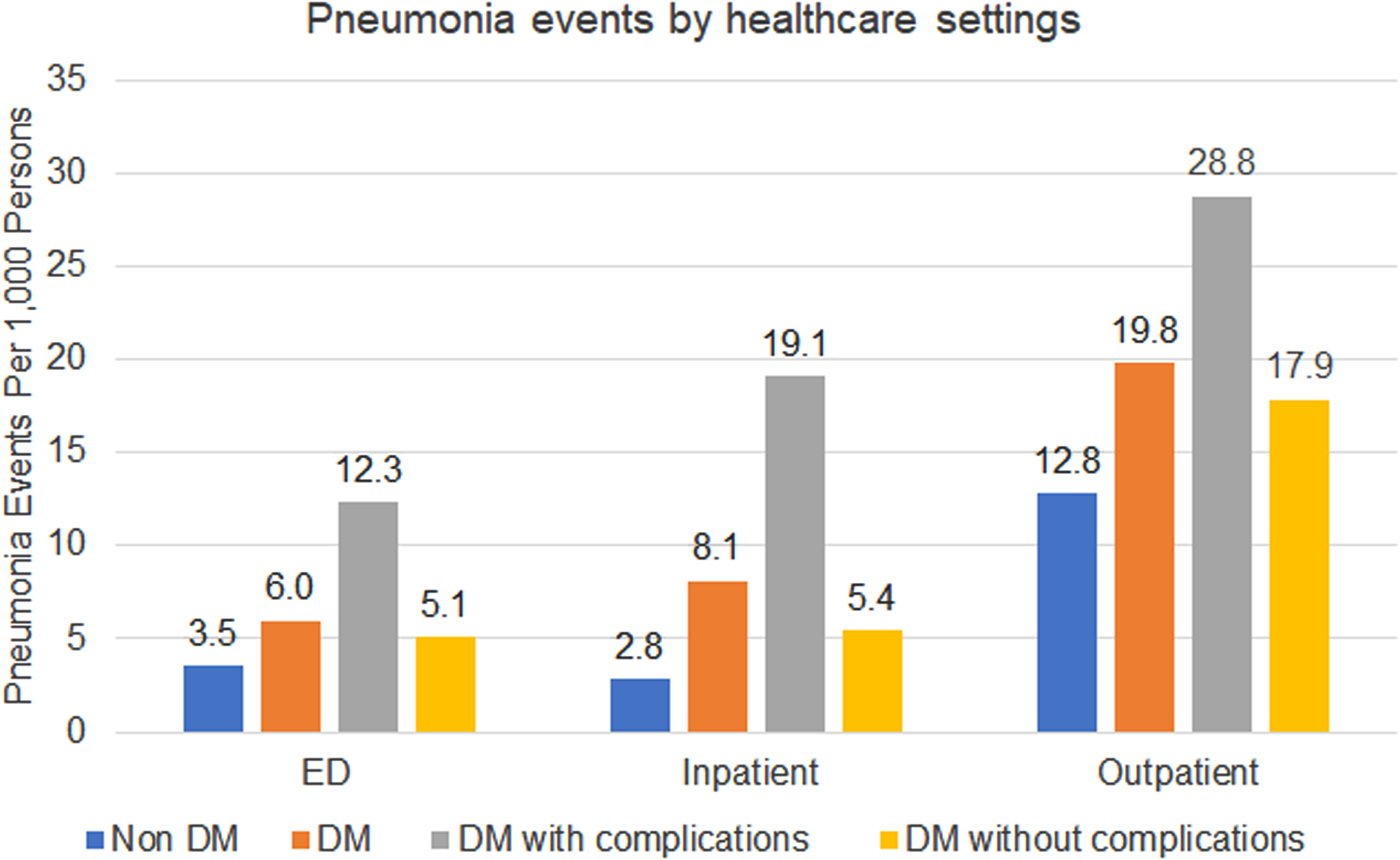
Fig. 1. Pneumonia events by healthcare settings.
The annual total expenditure for pneumonia events in 2014 was $3 billion and $9 billion in the DM population and the non-DM population, respectively. This was mostly driven by inpatient costs, comprising 89% of all expenditures. The average cost per pneumonia event was higher among DM compared to non-DM in the inpatient setting ($11 931 vs. $7751; P < 0.001). Additionally, the average cost expenditures per pneumonia event were similar when managed in the outpatient ($344 vs. $87; P = 0.314) and ED ($1072 vs. $883; P = 0.449) for DM and non-DM individuals, respectively.
Discussion
This large cross-sectional study describes the pneumonia prevalence, risk factors and healthcare utilisation patterns for individuals with DM in the USA. This study demonstrated that individuals with DM, especially those with DM-complications, have a higher likelihood for being treated with pneumonia and are associated with a disproportionate economic burden.
Individuals with DM are considered at higher risk for infections [Reference Peleg4, Reference Casqueiro and Alves6, Reference Muller8]. In a systematic review, individuals with DM were found to have a 40% higher risk for community-acquired pneumonia (CAP) and 2.3-fold higher risk for pneumococcal pneumonia [Reference Torres5]. In a study conducted by The Emerging Risk Factors Collaboration, it was found that individuals with DM had a 1.7x higher mortality risk from pneumonia compared to those without DM [9]. Yende et al. determined that patients with DM had a higher risk of mortality post-CAP [Reference Yende10]. Finally, Martins et al. demonstrated that DM patients had a higher prevalence, longer hospital stay and higher risk of mortality from CAP than non-DM patients [Reference Martins11]. Our study findings further support the notion that pre-existing DM is associated with a higher risk of acquiring pneumonia. This study also identified that the population rate for pneumonia among those with DM complications was doubled compared to those without complications, implying that DM complications may be an additional risk factor for pneumonia in this population.
Furthermore, this study found that individuals with DM had a higher rate of seeking care for pneumonia compared to individuals without DM across all three treatment settings. The primary setting for the management of pneumonia was in outpatient clinics, comprising of ~70% of all visits for pneumonia. However, individuals with DM were more likely to be hospitalised for pneumonia compared to non-DM. When examining individuals with DM, those with DM-complications had higher rates of seeking care for pneumonia across all treatment settings. Furthermore, the estimated total annual expenditure for pneumonia was $12 billion in 2014 with ~90% driven by inpatient costs. A 2007 retrospective database analysis found that the presence of DM was associated with a 70% higher average healthcare cost for pneumonia. Inpatient DM patients incurred an additional $8404 compared to its non-DM counterparts [Reference Polsky, Bonafede and Suaya12]. A 2008–2014 commercial claim study determined that the overall mean pneumonia hospitalisation cost was $10 963 [Reference Tong13]. Comparatively, this study describes a disproportionately higher average cost per pneumonia hospitalisation among DM compared to non-DM.
This study identified female sex as a risk factor for pneumonia among individuals with DM. Past studies evaluating sex as a risk factor for pneumonia are limited and inconsistent. Recent literature suggests that male sex increases the incidence and severity of pneumonia. Male patients have demonstrated to have a higher risk for lower respiratory tract infections (LRTIs) and higher hospital mortality from pneumonia [Reference Falagas, Mourtzoukou and Vardakas14, Reference Raine15]. The pneumonia severity indices include male sex as a risk factor in assessing the mortality risk and disposition [Reference Fine16]. However, these studies do not account for other factors including underlying effects of DM and sex [Reference Kaplan17]. Manicardi et al. found that women with DM had consistently higher haemoglobin A1c than males despite the same treatment intensity and goals, suggesting a pathophysiological difference between sex [Reference Manicardi18]. Further studies are needed to understand potential sex differences in DM and risk for pneumonia [Reference Regitz-Zagrosek19].
Factors associated with DM-complications were found to be associated with pneumonia among individuals with DM. Kornum et al. identified that having DM for greater than 10 years was associated with a 1.4x risk of having a pneumonia-related hospitalisation [Reference Kornum20]. Studies evaluating infection risk among those with DM-complications, primarily those with diabetic nephropathy, have shown that the presence of DM-complications is associated with higher pneumonia incidence rates and poorer prognosis [Reference Huijts21, Reference Slinin, Foley and Collins22]. Our study further supports that individuals with DM-complications are more likely to be hospitalised. Together, these findings suggest that targeted initiatives for this population are needed due to their significantly higher risk for pneumonia. Current pneumonia risk assessment scores do not factor in DM, nor associated complications, into determining risk and treatment [Reference Fine23].
A large number of studies have demonstrated that preventative pneumococcal and influenza vaccination improved clinical and economic outcomes, particularly among DM individuals and older adults [Reference Zhang24–Reference Fisman28]. However, vaccination rates continue to remain suboptimal. We found that only 49% of adults reported receiving an influenza vaccine during the past year and 61% reported receiving a pneumococcal vaccine. These rates are consistent with those described in other reports ranging from 20% to 38% for high-risk groups under the age of 65 years and 60–72% for those over the age of 65 years [Reference Williams29, Reference Lu30]. These low vaccination rates are likely due to multiple factors including misconceptions and lack of education about pneumococcal vaccinations among providers and the public [Reference Jones31]. A recent survey administered by the American Diabetes Association showed that only 35% of DM individuals believed that they were at high risk for pneumonia. This underscores a greater need for proper patient education [Reference Eisele32].
There were limitations to this study. First, we used CCS codes to identify pneumonia occurrences. Relying on coding for pneumonia rates is inherently subject to bias introduced by potential misclassification and coding errors. While LRTIs are frequently managed by nurse practitioners, physician assistants and other non-physician clinicians, this sample did not include non-physician clinician office-based visits. This might have underestimated the true burden of pneumonia in the ambulatory or outpatient setting. Moreover, this was a cross-sectional study design and might be susceptible to ecological fallacy bias. The prevalence data were based on total events and does not provide patient-specific information. Therefore, it is impossible to determine disease severity, microbiological aetiolgy, or other radiology or laboratory values to validate the diagnosis of pneumonia. Other potential confounders not available in this dataset were not considered in the analysis. However, to the best of our knowledge, this is the first large study to provide a holistic perspective of the burden of pneumonia and the care-seeking patterns in individuals with and without DM in the USA. This study also provides insights into previously unconsidered factors for individuals with DM who experience pneumonia.
In conclusion, individuals with DM have a higher risk for increased healthcare utilisation patterns compared to non-DM individuals for the management of pneumonia. Further studies are needed to examine specific risk factors for this population and their impact on pneumonia treatment decision-making and infection prevention efforts.
Author ORCIDs
G. C. Lee, https://orcid.org/0000-0002-7653-3028.
Financial support
This study did not receive any funding.