Introduction
Noroviruses are non-enveloped RNA viruses of the Caliciviridae family which have six genogroups (GI to GVI), although only genogroups I, II and IV are human pathogens [Reference Atmar, Ramani and Estes1, 2]. Norovirus infection is usually characterised by nausea, vomiting and a self-limiting evolution of about 48–72 h [Reference Heyman3].
The viral capsid provides specific resistance to the external environment, including high levels of chlorine [Reference Keswick4], heat and cold [Reference Butot, Putallaz and Sánchez5], acidic pH and organic solvents [Reference Butot6, Reference Green, Knipe, Howley, Cohen, Griffin, Lamb, Martin, Racaniello and Roizman7]. These characteristics mean norovirus is highly infective and can survive a long time in the environment [Reference Wu8]. There are different modes by which transmission may occur (direct person-to-person or indirect via faecal contamination in the environment or on foods) [Reference Torner9]. The low infectious dose (mean of 18 viral particles) [Reference Green, Knipe, Howley, Cohen, Griffin, Lamb, Martin, Racaniello and Roizman7], and the lack of long-term immunity in infected persons (the duration of immunity has been considered to be 6–24 months, although a recent study suggests that immunity lasts 4–9 years following infection) [Reference Ramani, Estes and Atmar10], means that norovirus often causes outbreaks in institutions and closed and semi-closed groups.
Norovirus is the main cause of viral acute gastroenteritis (AGE) outbreaks worldwide (>90% of AGE outbreaks are attributed to norovirus) [Reference Patel11], causing >1.8 million deaths in children aged <5 years annually [Reference Bryce12].
As in other European countries [Reference Friesema13], in Catalonia norovirus outbreaks are frequent in nursing homes: in 2010–2011 nearly 30% of norovirus outbreaks occurred in this type of setting [Reference Torner9], where older age and multiple comorbidities can result in hospital admission and even death [Reference Desai14]. The immediate introduction of infection control measures (hand hygiene, principles of food safety, disinfection of contaminated surfaces, isolation of ill persons and no return to work until 2 days after the absence of symptoms) decreases the duration of outbreaks and the attack rates in residents and staff [Reference Friesema13].
In Spain, the institutionalised population increased by >90% between 2001 and 2011, and more than 60% of this population (2 70 000 people) are estimated to live in nursing homes [15].
Many studies have looked at the factors that cause outbreaks, but fewer have looked at transmission beyond the primary outbreak setting [Reference Marsh16–Reference Gastañaduy19]. The objective of this study was to investigate a foodborne outbreak of norovirus in a nursing home and measure its impact on residents and the spread in staff and their household contacts.
Material and methods
Outbreak notification
On 16 March 2018, a nursing home notified a possible AGE outbreak that affected nine residents and two staff. The onset of AGE in the first cases occurred on the afternoon of March 15. The predominant symptoms were nausea, vomiting and watery diarrhoea without blood or fever. When the suspected outbreak was notified, some affected individuals were already recovering and no patient required hospitalisation
Outbreak setting
The care home had 166 residents on three floors. Residents with greater autonomy (116) lived on the first floor, semi-autonomous persons (27) on the second floor and those with the highest degree of dependency (23) on the third floor. The home had 119 staff: 42 on the first floor (three shifts of 14), 12 on the second floor (three shifts of 4) and 12 on the third floor (three shifts of 4). The remaining 53 staff (maintenance, laundry and cleaning, kitchen and medical) were not assigned to a specific floor.
The centre had a kitchen and dining room on the ground floor that was used by patients admitted to the first and second floors. On the third floor, there was a second dining room for patients although they could have meals in their rooms.
Various menus were prepared according to individual characteristics (normal diet, diabetic, easy chewing, soft foods and low calorie), although the menus were basically combinations of different dishes and only the soft food menu was markedly different.
The home had been in operation for some years; the structure and facilities were adequate for its stated activity and the number of residents was below the maximum capacity. Routine inspections had detected no deficiencies.
Definitions
A clinical case of norovirus infection was defined as a person with onset of diarrhoea and/or vomiting between March 15 and 31 who lived or worked in the nursing or was a household contact of a staff member. A confirmed case was defined as a clinical case that was positive for norovirus in faeces by real-time polymerase chain reaction (RT-PCR).
Study phases
The study was carried out in three phases: The first, carried out on March 17 and 18, was aimed at verifying the aetiology and mechanism of the outbreak. An epidemiological survey was designed to gather socio-demographic data, symptom onset and end and clinical data. A food survey was conducted to determine the type of menu and the specific dishes consumed. The questionnaire was filled in by the health staff of the centre. A case–control study was designed: cases were chosen from clinical cases in residents and controls were chosen from residents who did not become ill.
The second phase, carried out from March 19 to 25, was aimed at quantifying the size of the outbreak and monitoring its evolution, in addition to maintaining the control measures indicated. Information on the demographic and clinical variables of new cases was collected. The health personnel of the home collected the information. The food-handling facilities were inspected, including collecting environmental samples from the kitchen and common use areas. It was not possible to process samples of the food potentially implicated in the outbreak as our laboratory does not have approved International Organization for Standardization certification for the food that the case–control study showed was associated with the outbreak.
Between March 26 and April 1, the extent of the outbreak in household contacts of staff was quantified. We actively investigated how many staff were clinical cases using a survey that included age, sex, whether they had been infected (with date of onset and end), number of household contacts of staff (with age and sex), whether these were clinical cases and date of onset. The aim of this survey, which was self-completed by staff, was to determine the extent of illness in staff and their household contacts.
Laboratory analyses
Stool samples were collected on March 18. Samples were sent to the Vall Hebrón University Hospital microbiology laboratory for testing for norovirus (if negative, testing for rotavirus, adenovirus, astrovirus and sapovirus was made). Given the suspicion of viral AGE, bacterial tests were not carried out. The specific primers described by Kageyama et al. [Reference Kageyama20] for the detection of norovirus GI and GII were used for RT-PCR. Norovirus genogroup IV (GIV) was detected using a modification of the primers described by Farkas et al. [Reference Farkas21] and Kageyama et al. [Reference Kageyama20]. For genotyping, the ORF1–ORF2 junction was sequenced from all samples laboratory-confirmed for norovirus. To amplify this region, a one-step RT-PCR assay was carried out using the One-Step RT-PCR kit (Qiagen, Hilden, Germany) with previously published primers GISKF, GISKR for GI NoV, and G2SKF and G2SKR primers for GII NoV [Reference Kageyama20, Reference Kojima22]. PCR products were then purified using Exo-SAP-IT (USB, Affymetrix Inc., Cleveland, Ohio, USA) and sequenced by the ABI Prism Big Dye Terminator cycle sequencing kit v3.1 on the ABI PRISME 3130XL sequencer (Applied Biosystems, Foster City, California, USA). Nucleotide sequences were assembled and edited using SeqMan 4.05 (Dnastar, Madison, Wisconsin, USA). Norovirus typing tool v2.0 was used to genotype norovirus.
Environmental samples were collected with polyester swabs. Total nucleic acids were extracted with BioMérieux NucliSENS easyMag system. The reagents, primers and cycling conditions of the real-time RT-PCR used for the detection of norovirus GI and GII were those indicated in ISO 15216-2:2013 [23].
The data were stored and subsequently debugged in a Microsoft Access database.
Statistical analysis
The mean age of clinical cases and controls was compared using the two-sample t test and percentages of the distribution of sexes using the χ 2 test. Univariate analysis was used to determine the associations between the consumption of each dish served and AGE, using the odds ratio (OR) and 95% confidence intervals (95% CI).
The risk of AGE in household contacts of staff who were clinical cases or not was calculated using the rate ratio (RR) and 95% CI.
In staff, the association between professional category and the risk of AGE was estimated by calculating the OR and 95% CI. In household contacts of staff that were clinical cases, the risk of infection and the determining factors were estimated by calculating the OR and 95% CI. Multivariate logistic regression was carried out to control for the influence of possible confounding variables: the variables age of household contact, sex of household contact, number of household contacts and the duration of illness in staff were included in the adjustment. The statistical analysis was made using the PASW Statistic 18.0.2 statistical package.
Results
Information was collected on 361 people (54 residents, 119 staff and 188 household contacts of staff). Seventy-nine persons (25 staff and 54 residents) were exhaustively surveyed, of whom 56 were infected and 23 were not. For the remaining 94 staff and 188 household contacts of staff, socio-demographic information, professional category and, in clinical cases, the date of onset, was collected.
The AGE outbreak affected 137 people (39 residents, 55 staff and 43 household contacts). The patients affected were mainly residents of the first and second floors and some staff, with no resident of the third floor being affected. The temporal grouping of the first reported cases suggested a common exposure that might have occurred at dinner on March 14, although breakfast on March 15 could not be ruled out. The clinical characteristics suggested norovirus as the cause of the outbreak.
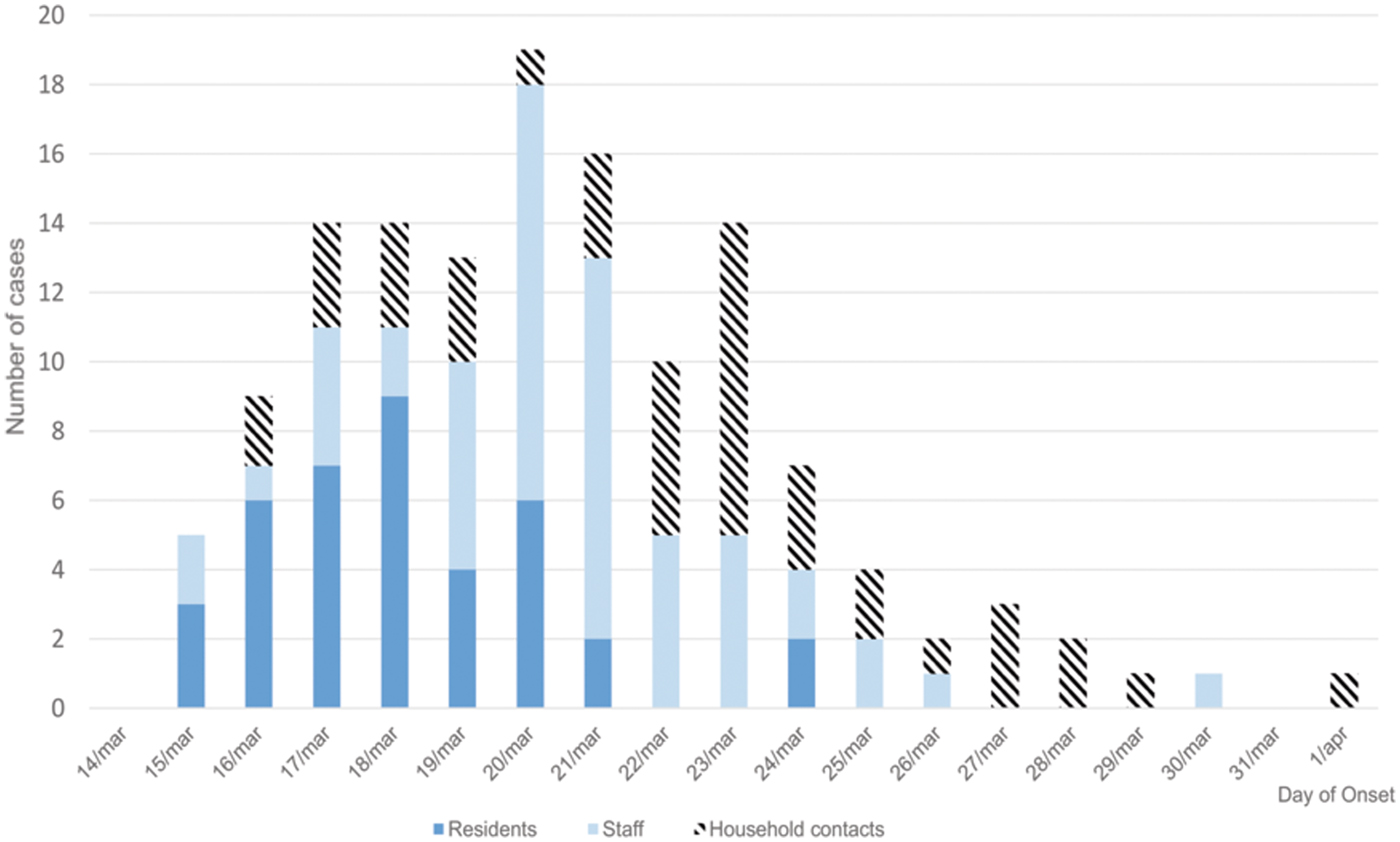
Figure 1 shows the epidemic curve, and clinical cases in residents, staff and household contacts.
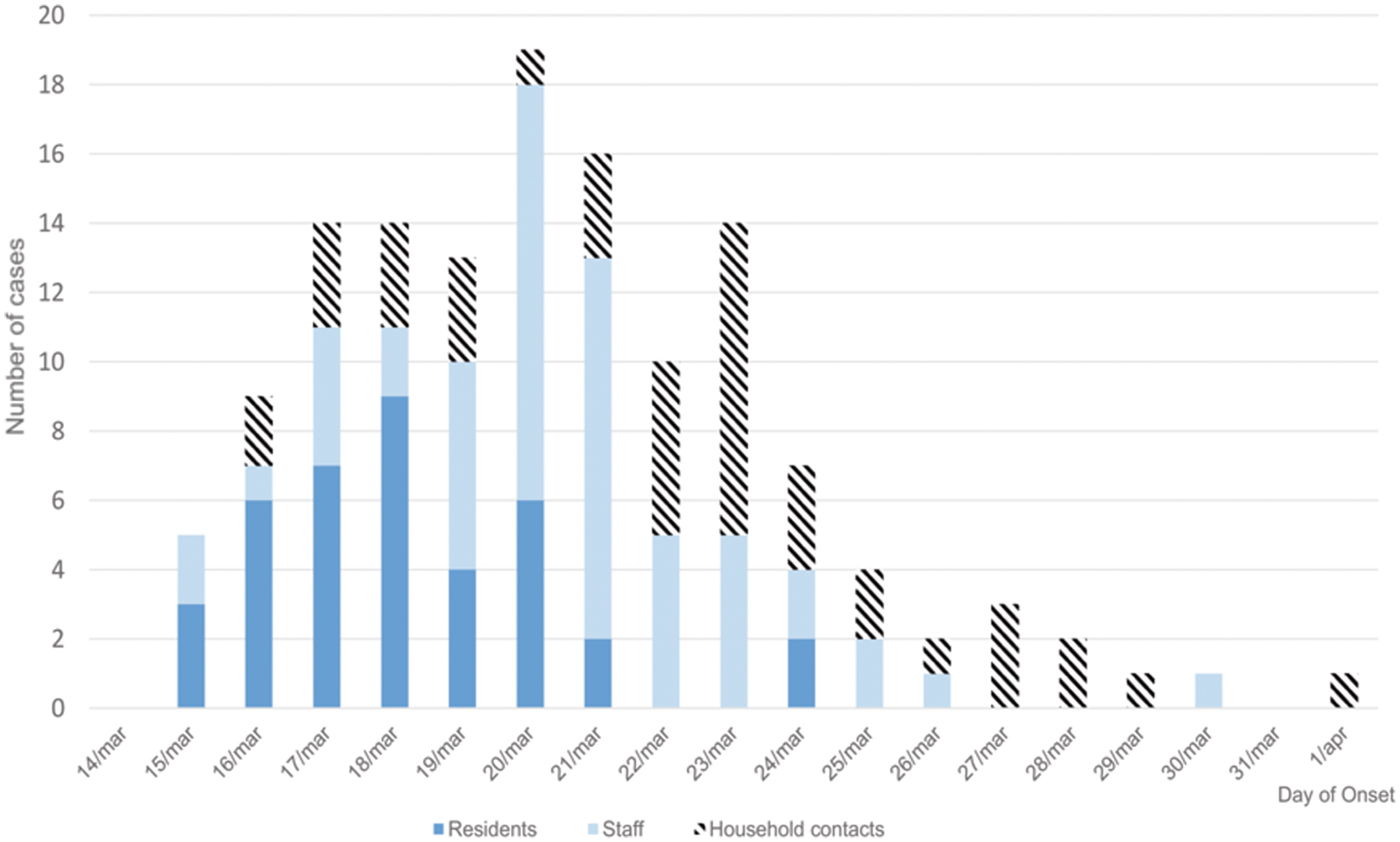
Fig. 1. Distribution of clinical cases by day of onset in residents, staff and household contacts.
The attack rates in residents, staff and household contacts were 23.49%, 46.22% and 22.87%, respectively. The most frequent symptoms were vomiting (64.3%), diarrhoea (62.5%), abdominal pain (37.5%) and fever (14.3%). Symptom duration was approximately 24 h (median 1 day, range 1 h 55 min to 4 days).
Stool samples were collected from 28 people: 14 were clinical cases (10 residents and four caregivers) and 14 were not (six residents, three caregivers and five kitchen staff). Ten samples were positive for norovirus (two GI, six GII and two GI/GII). The genotype was identified in nine cases: seven were GII.17 and two GI.3. Six positive samples came from confirmed cases and four from asymptomatic persons. Eight environmental samples were collected (three from kitchen staff lavatories, two from the kitchen and three from common areas of the residence). No environmental sample was positive for norovirus.
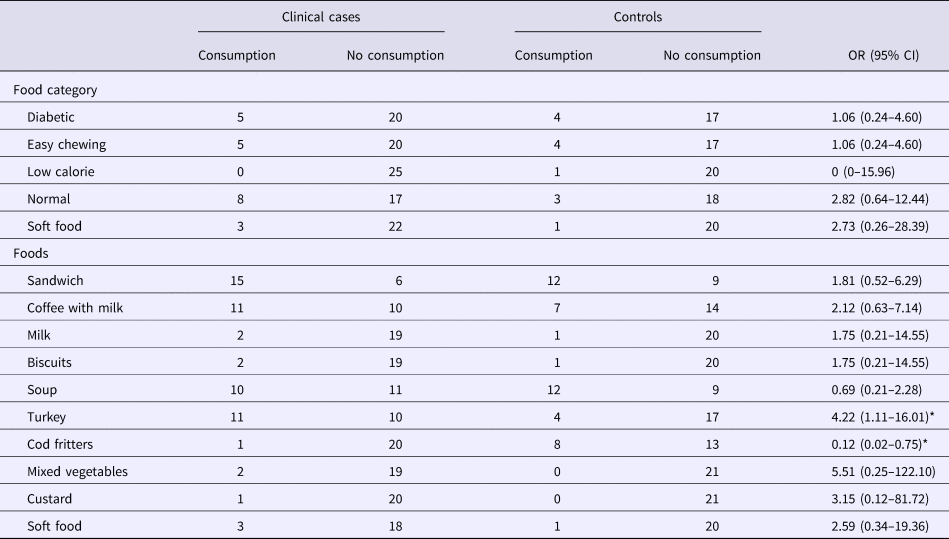
The food consumption questionnaire was answered by 46 residents (25 clinical cases and 21 controls), including the type of diet and the food served during the two suspect meals. In four people, it was only possible to identify the type of diet followed. Twenty per cent of clinical cases and 19% of controls were male: the mean age was 78.8 years (s.d. 22.4) in clinical cases and 71.2 years (s.d. 26.2) in controls: the differences were not statistically significant. Table 1 shows the distribution of individuals who were clinical cases or not, according to the consumption of a specific food or diet. No significant differences were observed between different diets. However, when the consumption of each food was analysed, the turkey served on the night of March 14 was associated with being a clinical case (OR 4.22, 95% CI 1.11–16.01). In contrast, the alternative to turkey (cod fritters) showed an OR of 0.12 (95% CI 0.02–0.75).
Table 1. Distribution of clinical cases and controls according to food consumption

OR, odds ratio.
*Statistically significant values (P < 0.05).
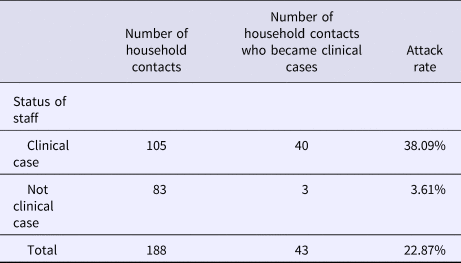
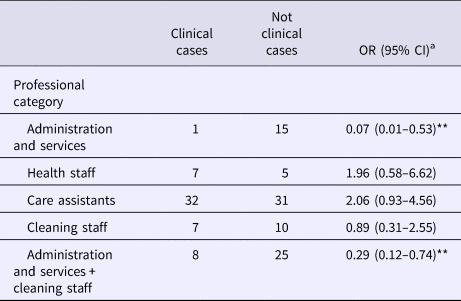
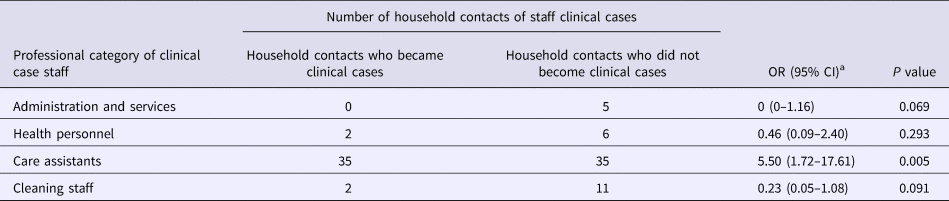
Table 2 shows the attack rate in household contacts of staff that were clinical cases or not. The overall attack rate in household contacts was 22.87%, although the rates clearly differed between household contacts of staff with and without illness (38.09% vs. 3.61%), with an RR of 10.54 (95% CI 3.38–32.87; P < 0.001). Table 3 shows the risk of being a clinical case in each professional category, expressed as the OR and 95% CI. The administration and services categories had a protective effect (OR 0.07, 95% CI 0.01–0.53). Table 4 shows the risk of being a clinical case in household contacts in each staff category, expressed as the OR and 95% CI and the level of significance. Household contacts of a care assistant had a 5.5-fold higher risk of being a clinical case than household contacts of the remaining staff. The professional categories of the remaining staff did not influence the risk of their household contacts being a clinical case. In the multivariate analysis (Table 5), the administration and service categories were too small for separate analysis and were therefore grouped with the cleaning staff. After adjustment, the only factor significantly associated with the risk of being a clinical case was being a household contact of a care assistant (adjusted OR 6.37, 95% CI 1.13–36.02).
Table 2. Attack rates in household contacts of staff according to clinical case status in staff
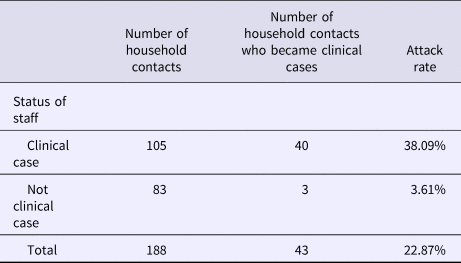
Rate ratio (RR): 10.54 (95% CI 3.38–32.87; P < 0.001).
Table 3. Clinical cases according to professional category

OR, odds ratio.
a Comparing each professional category with the rest.
**Statistically significant values (P < 0.05).
Table 4. Risk of becoming a clinical case in household contacts of clinical cases in staff
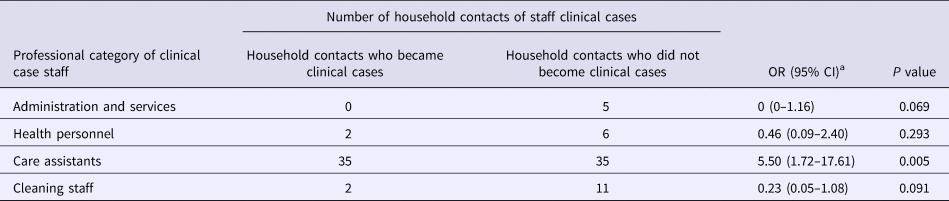
OR, odds ratio.
a Comparing each professional category with the rest.
Table 5. Risk of becoming a clinical case in household contacts of the clinical cases in staff. Multivariate analysis

aOR, adjusted odds ratio; Ref., reference group.
a Adjusted for age of household contact, sex of household contact, number of household contacts and duration of illness in staff.
Discussion
Our results show that the AGE outbreak investigated originated from common exposure to food and was subsequently spread by person-to-person transmission. The epidemic curve showed the rapid emergence of cases, mostly in residents, and then a more pronounced peak including staff and spread to household contacts of staff. The distribution of clinical cases in residents was typical of the distribution of cases in a common source outbreak due to a specific exposure [Reference Fontaine, Goodman and Gregg24].
Reports have described outbreaks that begin with food poisoning and continue with person-to-person transmission in nursing centres and other institutions. Becker et al. described an AGE outbreak due to the consumption of turkey sandwiches in a football team and transmission to the opposing team [Reference Becker25]. Marsh et al. analysed three outbreaks of food poisoning in which secondary cases due to person-to-person transmission were found in household contacts of persons affected: 25% of the homes of those affected had secondary cases, with an attack rate of 20% among contacts [Reference Marsh16].
The epidemic curve in staff showed a delay in the onset of illness compared with residents, which suggests residents were involved first and there was secondary transmission to staff. Cases in staff from the beginning of the outbreak suggest that, although the staff did not have dinner in the centre, some probably consumed the foods that triggered the outbreak. A possible reason for the absence of clinical cases in third floor residents may be explained by the fact that these persons did not consume the food that caused the outbreak and, due to limitations in movement, did not have physical contact with the other residents.
Norovirus GI and GII co-infection was detected in two patients. Co-infection by more than one genogroup during epidemic norovirus outbreaks is not exceptional. Ushijima et al. in a review of food poisoning due to norovirus in Japan found GI/GII co-infection in up to 10% of outbreaks [Reference Ushijima26]. Huang et al. analysed an outbreak that affected six groups of tourists and, of the 23 stool samples analysed, 22 were positive for norovirus GI and/or GII (six patients were co-infected by the two genogroups) [Reference Huang27]. The presence of more than one norovirus genogroup has also been observed when possible sources of infection are analysed. In an outbreak of AGE due to norovirus whose origin was bottled mineral water distributed among 925 Spanish companies, both the analysis of the water and the faeces of affected people were positive for norovirus GI and GII [Reference Blanco28].
The attack rates in staff (46.22%) and residents (23.49%) are those expected in a norovirus outbreak due to food in a nursing home with these characteristics. A review by Lindsay et al. of norovirus infection in older people from middle- and high-income countries found the attack rate in outbreaks in nursing homes ranged from 3% to 45% [Reference Lindsay29]. Similar variability was found by Green et al. in a study of 20 AGE outbreaks between 1987 and 1988, with the attack rate ranging between 5% and 59% (median 27%) in residents of nursing homes and between 0.6% and 26% (median 9%) in staff [Reference Green30]. Utsumi et al. in another review of outbreaks of infectious aetiology in long-stay centres between 1966 and 2008 found that, in norovirus outbreaks, the mean attack rate was 45% (range 13–100%) in residents and 42% (range 9–100%) in staff [Reference Utsumi31].
We found an attack rate in household contacts of clinical cases in staff of 22.87%, similar to the results of other studies. Marsh et al. described a secondary attack rate of 20% in household contacts of infected persons in three outbreaks of food poisoning [Reference Marsh16]. In an outbreak of food poisoning due to norovirus related to the consumption of oysters in a restaurant in North Carolina in 2009, secondary cases were found in 20% of households included in the analysis and the attack rate was 14% in household contacts (who had not eaten in the restaurant) [Reference Alfano-Sobsey17]. Heun et al. studied a 1984 outbreak of food poisoning in a school due to norovirus and, as in our study, the risk in household contacts of those affected was greater than the risk in household contacts of persons who did not become ill [Reference Heun18].
Although the association was not significant, we found the most frequently affected staff were care assistants (OR 2.06, 95% CI 0.93–4.56), who have the most direct, closest and longest contact with residents. The intensity of contact plays a fundamental role in the risk of infection during an AGE outbreak due to norovirus. Petrignani et al. reviewed norovirus outbreaks in care homes and found a higher intensity of contact between staff and residents was associated with higher attack rates [Reference Petrignani32]. Godoy et al. found attack rates of 56.5% in care assistants in an outbreak in an assisted living facility [Reference Godoy33] and González Moran et al. found attack rates of 35.8% in health staff (mostly care assistants) in a nursing home [Reference González Morán34]. These two studies also found that cleaning staff were frequently affected (attack rates of 55.6% and 40.0%, respectively). In our study, the attack rate in cleaning staff was 41.2%, similar to other studies [Reference Godoy33, Reference Mayoral Cortés35]: administration and service staff were the least affected (attack rate of 6.67%), and this category was a protective factor against infection (OR 0.07, 95% CI 0.01–0.53).
The greater involvement of care assistants could be due in part to the pressure of care to which they are subjected. According to a 2011 report by the Organization for Economic Cooperation and Development (OECD), the percentage of staff assigned to care for older people is below what is desirable, but in some countries, such as Spain, this is especially relevant. While Sweden allocates 3.6% of staff to this sector, Spain allocated just over 1% [Reference Colombo36].
There were also AGE cases in household contacts of staff, with a higher attack rate in people who lived with infected staff than in those who did not (38.09% vs. 3.61%). This result is consistent with the study by Sukhrie et al. of five outbreaks of AGE due to norovirus in two care homes and a university hospital in Rotterdam, which described the role of health staff in the transmission of the virus, while noting the low importance of asymptomatic carriers [Reference Sukhrie37]. There was a significant association between the risk of household contacts of care assistants becoming infected and the professional category of the worker (OR 5.50, 95% CI 1.72–17.61).
Multivariate analysis showed that household contacts of care assistants had an increased risk of infection (adjusted OR 6.37, 95% CI 1.13–36.02), independently of other variables such as the number of people in the home, the age or sex of household contacts and the duration of symptoms presented by staff.
The study had some limitations. First, we could not confirm the greater risk of illness among cleaning staff, probably due to a lack of statistical power. Second, the number of clinical samples did not allow to study the possible differences between people affected by GI, GII or co-infected by both genogroups. Third, we could not analyse the presence of norovirus in the foods involved in the outbreak, especially in the turkey dish served at dinner on March 15, which was statistically associated with the appearance of AGE. Fourth, clinical samples were not available for household contacts that became clinical cases; however, the attack rates of household contacts of ill and unaffected staff showed significant differences, suggesting that cases in household contacts of staff were related to the outbreak and not to other viruses circulating in the community.
In conclusion, our results suggest that the study of AGE outbreaks in nursing homes may be extended to the household contacts of staff. Staff that have more intense contact with residents, such as care assistants, are more likely to generate secondary cases in household contacts, which has important health and economic consequences [Reference Bartsch38, Reference Navas39]. Care assistants working in nursing home staff should receive additional training in the application of preventive protocols.
Author ORCIDs
I. Parrón, 0000-0002-4875-0236; À. Domínguez, 0000-0003-0219-1907.
Financial support
This study was funded by the Carlos III Health Institute through the project PI16/02005 (Co-funded by the European Regional Development Fund ‘Investing in your future’) and the Catalan Agency for the Management of Grants for University (AGAUR Grant Number 2017/SGR 1342).
Conflict of interest
None.