Introduction
The spread of carbapenemase-producing Enterobacteriaceae (CPE) is a current threat to patient safety worldwide. The recent emergence of these highly resistant bacteria is a public health concern as CPE infections are associated with higher morbidity and mortality, longer hospitalisations and higher costs [Reference Nordmann and Poirel1, Reference Lee2].
OXA-48 is a carbapenemase belonging to Ambler class D beta-lactamases, identified frequently in the Mediterranean and Southern European countries. Firstly reported in 2001 in a hospitalised patient in Turkey, it rapidly spread and currently is a frequently reported hospital and community-acquired resistance associated to several Enterobacteriaceae species [Reference Girmenia, Serrao and Canichella3, Reference Nordmann4].
In 2012, the Centers for Disease Prevention and Control (CDC) published a specific guide for CPE control [5]. In Europe, specific CPE plans to control outbreaks in healthcare institutions started to be implemented in 2013 [6, 7].
However, despite the efforts made during years trying to contain the advance of CPE, data published by international organisations are not very encouraging [8, 9].
Among all the recommendations, active surveillance for early detection of colonised patients in addition to enhanced infection control practices, has been the cornerstone of the successful containment of some hospital outbreaks [Reference Schwaber and Carmeli10–Reference Kochar13].
In 2013, the Spanish background changed from a country with sporadic hospital outbreaks of OXA-48-PE to a country with the regional spread. Our hospital was among the firsts in Spain to recognise this problem and try to quantify the magnitude of clinical infections [Reference Paño-Pardo14, Reference Fernández15].
The aim of this report was to describe the epidemiological and microbiological data of patients in whom an OXA-48-PE was isolated in our hospital between January 2013 and December 2014, to describe the prevention and control measures implemented, and the OXA-48-PE CI trends.
Methods
Setting
La Paz University Hospital (LPUH) is a tertiary hospital with 1300 beds for acute care which provides service to a population of 600 000 people of whom 14.5% are over 65 years. It is a hospital of a high complexity with six buildings and an average of 46 300 admissions per year. In 2014 Hospital Carlos III joined LPUH, representing an increase of 465 admissions with respect to the previous year.
Definition and extent of the outbreak
In December 2010, we detected the first patient infected with OXA-48-PE. During 2011 and 2012, despite the efforts made, the outbreak could not be contained and small clusters in different hospitalisation units prolonged it.
Throughout 2013 new measures were implemented, such as the systematisation of rectal screening in inpatient units (weekly and at admission), strict patient and staff cohort in a separate ward, use of chlorhexidine soap for patient hygiene and reinforcement of the hospital ward cleaning.
Design and period
An observational retrospective study of microbiological and epidemiological data of OXA-48-PE cases between 01/01/2013 and 31/12/2014 was carried out. OXA-48-PE cumulative incidence (CI) trend was determined from January 2013 to July 2015.
Case description
Patients registered as ‘colonised’ had at least one sample of rectal colonisation with OXA-48-PE, and no clinical specimen isolated during the study period. Patients registered as ‘clinical sample’ were those in which it was isolated an OXA-48-PE in at least one clinical sample. One sample (the first one) per patient was included in the study for CI calculation, demographic characterisation and epidemiological and microbiological description of incident cases. When a patient presented another clinical specimen positive for OXA-48-PE in a different site than the one of the debut, that information was also recorded.
Inclusion criteria
Cases in which the first isolation with OXA-48-PE (either clinical or colonisation sample) was related to healthcare assistance in LPUH. We considered health care assistance in LPUH if they had been admitted at least once to our hospital during the previous year. Patients in ‘Emergency’ or ‘Outpatients’ were considered as related to healthcare in LPUH if they had received assistance in the Emergency Department more than 48 h during the previous year of the first isolation date; or if they had been in ‘outpatients clinics’, or receiving some kind of treatment in one of the ‘Day Hospital’ during the previous year of the first isolation date, though they had not been admitted at the hospital.
Exclusion criteria
Cases of isolation with OXA-48-PE (either clinical or colonisation sample) not meeting inclusion criteria were excluded from the study.
Data source
Clinical and demographic data were obtained from hospital records. Basic epidemiological data were daily recorded to manage outbreaks, the rest of variables were retrospectively obtained. Microbiological data were provided by the Department of Microbiology, and epidemiological data were processed and analysed by the Preventive Medicine Department.
Bacterial isolates and determination of extended spectrum beta-lactamase (ESBL) and carbapenemase production
Surveillance samples were cultured directly on MacConkey agar plates containing 4 mg/l cefotaxime (Tec-Laim, Madrid, Spain). Isolates were identified using a MALDI Biotyper (BrukerDaltonik, Bremen, Germany). Antibiotic susceptibility was determined using the Wider® system (Francisco Soria Melguizo, Madrid, Spain) and MicroScan® WalkAway (Beckman Coulter, Brea, CA, USA) and isolates were categorised as susceptible or resistant according to EUCAST V3.1. Carbapenemases and ESBLs were tested in Enterobacteriaceae by the modified Hodge test using imipenem, meropenem and ertapenem disks and by the double-disk diffusion method with cefotaxime/cefotaxime-clavulanic acid and ceftazidime/ceftazidime-clavulanic acid. MIC50 and MIC90 were defined as the lowest concentrations of antibiotic that inhibited 50 and 90% of the isolates respectively.
Characterisation of blaOXA-48 by PCR, DNA sequencing and molecular typing
Carbapenemase production was confirmed by PCR (Progenie Molecular, Valencia, Spain). The genetic relationships between the isolates were determined by automated repetitive-sequence-based PCR (rep-PCR) using the DiversiLab® (bioMérieux) system, then one or two representatives with the same rep-PCR pattern were screened by multilocus sequence typing (MLST). Whole genome sequencing of some isolates allowed the design of clone-specific real-time PCR for Klebsiella pneumoniae ST11 and ST405 [Reference Lopez-Camacho16]. When this clone-specific PCR was implemented for rapid typing, rep-PCR was only used for non-ST11 and non-ST405 clones.
Summary of the control measures implemented or reinforced during 2013
• Infection control measures: Reinforcement of contact precautions for all colonised/infected patients, fostering hand hygiene, improving the access to hand hygiene stations and identifying at re-admission those patients that had been previously colonised/infected by OXA-48-PE.
• Active surveillance: Weekly screenings and on admission screenings in critical care units (implemented previously and also maintained during the study period), and screening on admission and weekly in wards where transmission was detected. An active protocol for screening patients with epidemiologic links with carriers of CPE was also implemented.
• Reinforcement of environmental cleaning: Twice a day in CPE-carriers rooms using a chlorinated cleaning product. The chlorinated compound was previously tested in vitro to be effective against the two clones of OXA-48-producing K. pneumoniae, ST11 and ST405.
• Weekly meeting of a multidisciplinary experts group led by the Hospital Administration. Consisting of, among others, professionals from the Preventive Medicine, Microbiology, Infectious Diseases and Internal Medicine departments, this group had been meeting since 2011, but was formally established in March 2013, in order to be the executive body to implement the ‘CPE prevention strategies’.
• Healthcare Personnel Education: Multiple training sessions for all hospital staff. Also, an extraordinary training session led by the Managing Director was performed. Training in minimising use of invasive devices, hand hygiene and contact precautions were performed systematically in the Departments in which carriers were admitted and detected.
• Patient and Staff Cohort: In December 2012, an entire hospitalisation unit was set up as a cohort for transferable OXA-48-PE colonised/infected patients, with exclusive staff for their care.
• Chlorhexidine bathing: Patients baths with soap containing 4% chlorhexidine were implemented.
• Electronic alerts: In order to communicate the CPE status for infected and colonised patients at admission, electronic alerts were set up in the Emergency Department, and also for discharge and transfers to other facilities.
Statistical analysis
Quantitative variables were described as the mean and standard deviation (s.d.) or as median (percentiles). The qualitative variables were described as absolute and relative frequencies. SPSS, version 20 was used for analysis.
Joinpoint Regression Program was performed to analyse changes in CI trend of OXA-48-PE among January 2013–July 2015. Monte Carlo Permutation method was used to test significance.
Results
Outbreak and patients characterisation
The main epidemiological and microbiological features of the outbreak are shown in Table 1. During the study period (January 2013–December 2014), 816 incident cases were identified, 80% as a colonisation sample and 20% as a clinical specimen. In 15.6% of the 652 colonised patients, a clinical sample was isolated as a second specimen. In 32.5% of all patients, at least one clinical specimen was isolated too.
Table 1. Epidemiological and microbiological data of patients with OXA-48-PE
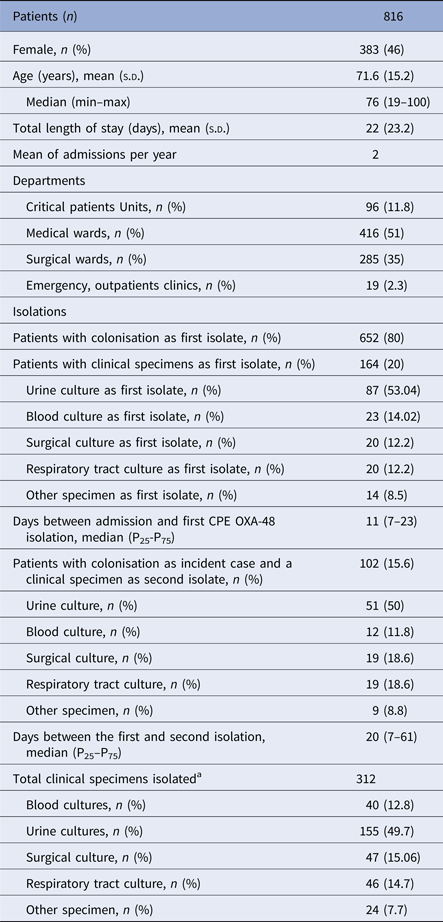
a Sum of first, second and following clinical specimens isolated in different sites per patient.
The most frequent clinical specimens identified as the first isolate were: urine (10.7%) followed by blood samples (2.8%).
The majority of patients were aged, had recurrent long admissions and more than a half were diagnosed in the medical wards with conventional hospitalisations (Haematology, Oncology, Geriatrics and Internal Medicine).
Two per cent of the 816 patients had no previous hospital admissions since 2010 but had some kind of healthcare related to the hospital (outpatients clinics or Emergency Department).
In Figure 1, the monthly epidemic curve is shown with incident cases according to first sample (colonisation or clinical sample) (a), and by healthcare areas (medical, surgical, critics and non-hospitalised) (b).
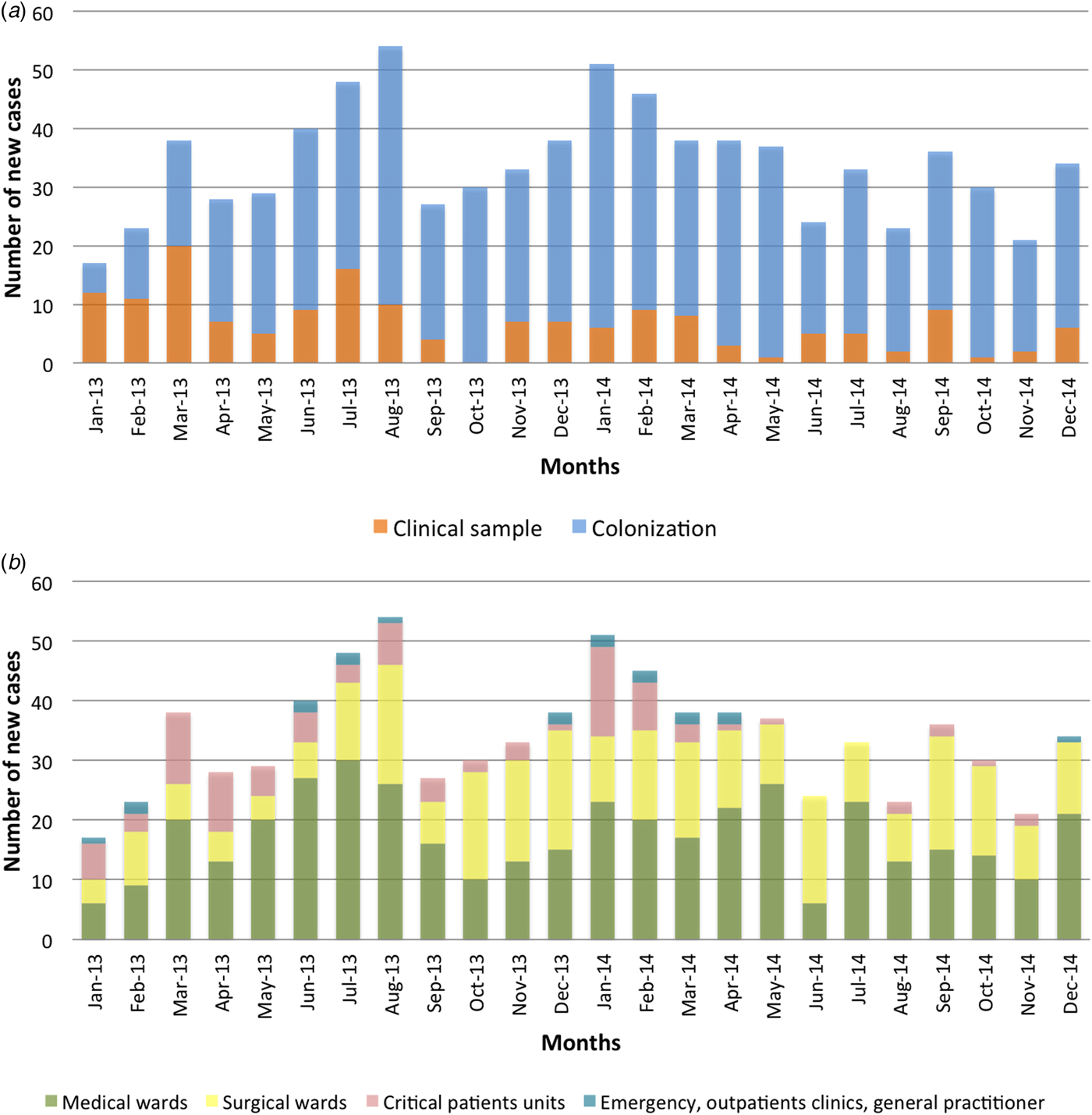
Fig. 1. Epidemic curve of new cases per month of OXA-48-PE according to the origin of sample (a) and according to ward of the patient (b).
Characterisation of OXA-48-producing Enterobacteriaceae
Species of OXA-48-PE isolated as incident cases were: K. pneumoniae (93.6%), Escherichia coli (2.3%), Enterobacter spp. (1.7%), both K. pneumoniae and E. coli (0.5%), and other species, like Raoultella spp. or Citrobacter spp. (1.8%) (Fig. 2).
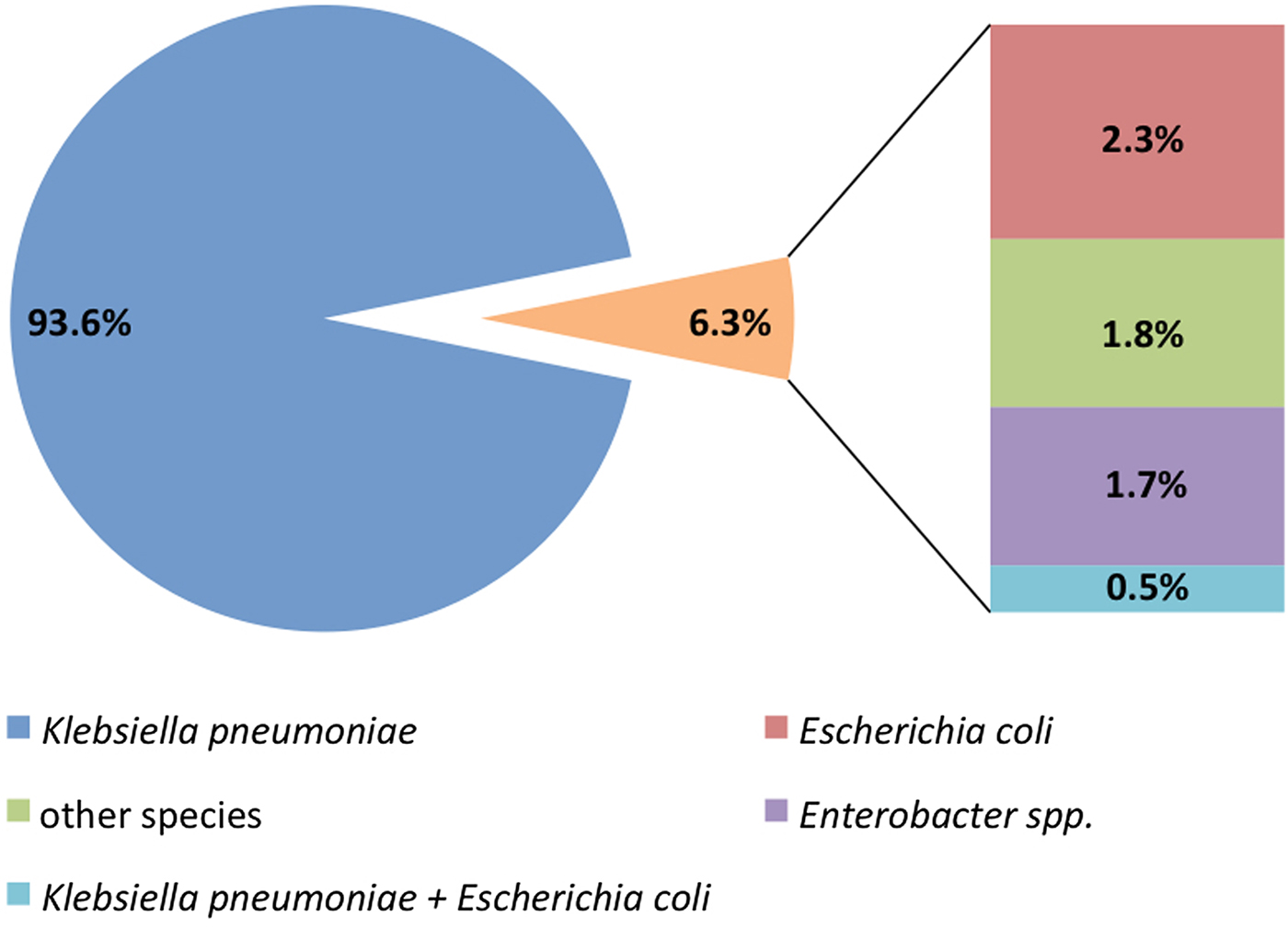
Fig. 2. Microorganisms isolated in the first sample with an OXA-48-PE.
Of the OXA-48-PE isolated from 266 clinical samples (164 clinical specimens as first isolate and 102 clinical specimens as the second isolate) and tested for antimicrobial susceptibility, 87.8% had a phenotype consistent with ESBL production. Accordingly, they were resistant to cefotaxime, ceftazidime, cefepime and aztreonam, and associated high-level resistance to amoxicillin/clavulanate and piperacillin/tazobactam. The MIC50 and MIC90 were 4 and 16 µg/ml for ertapenem, and 2 and 16 µg/ml for imipenem and meropenem. The isolates were resistant to all antibiotics tested with Wider® system and MicroScan® WalkAway panels except amikacin (94.8% of the strains tested were susceptible), tigecycline (83%), colistin (69.9%), fosfomycin (31%) and cotrimoxazole (14.8%).
Epidemiological pattern
The epidemiological pattern was very complex, consisting of the emergence of clusters in separated wards, frequently without an apparent link. It was also characterised by a continuous circulation and spread of three different clones of K. pneumoniae (ST11, ST405 and ST323) with sporadic appearance of up to eight different uncommon clones and occasional isolates of some other Enterobacteriaceae. K. pneumoniae sequence type 11 and 405 were predominant, representing 64.2% and 29.3%, respectively (Table 2).
Table 2. Distribution of sequence type (ST) of K. pneumoniae

OXA-48-PE CI trends
OXA-48-PE CI trends were analysed for the study period and extended to July 2015. Joinpoint regression shows a change from ascending to descending in August of 2013, with a slope change estimate of −15.2 and a P-value = 0.008. Slope 1 (from January 2013 to August 2013) presents a significant ascent (monthly percentage change of 14.3) with a P-value = 0.018. Slope 2 (from August 2013 to July 2015) shows a significant descent (monthly percentage change of −1.9) with a P-value = 0.022 (Fig. 3).
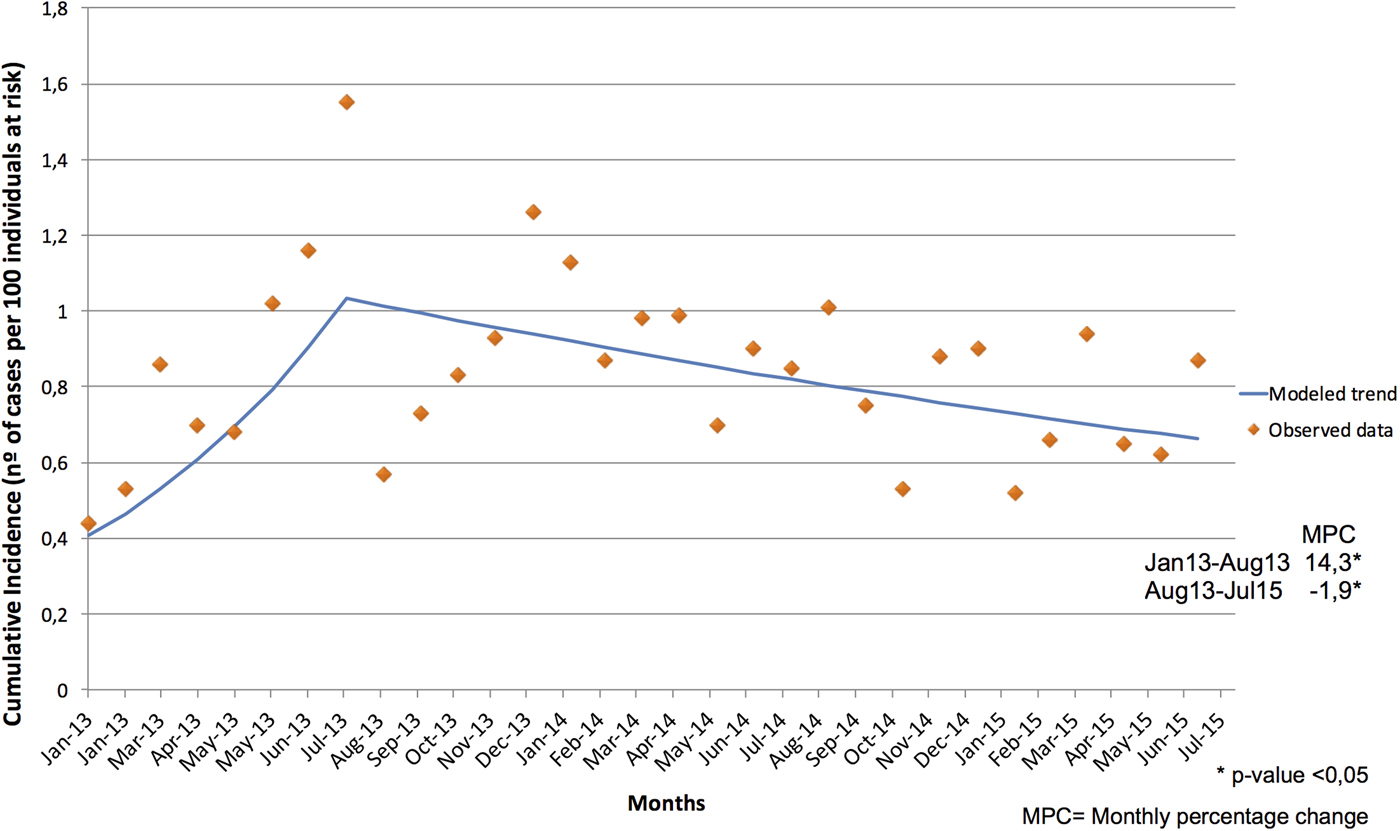
Fig. 3. Joinpoint Trend of Cumulative Incidence of OXA-48-PE from January 2013 to July 2015.
Discussion
The OXA-48-PE outbreak started in our hospital in January 2011. From the beginning, a disperse distribution was observed, quickly involving several departments [Reference Paño-Pardo14]. However, in spite of the implementation of standard control measures, the incidence kept increasing, with a predominant epidemic pattern that consisted of a cluster of limited outbreaks in different wards and in different buildings of the hospital, and mixed with a broad spatial distribution of sporadic isolates. The genetic studies carried out on our hospital strains, reflected an increase in the variability of the sequence types, but a predominance of ST11 in first place followed by ST405. These data match with the distribution described in Spain, where the inter-regional spread of ST11 and ST405 has been detected, and ST11 has successfully established during the last years [Reference Brañas17, Reference Pérez-Vázquez18].
Before the new interventions were launched in 2013, screenings were done systematically only in Critical Care and Haematology Departments so, outbreaks in other areas were discovered later and controlling them was difficult to achieve. In addition, there was no cohort area, so colonised/infected patients had to stay in individual rooms, or grouped in double rooms, but within the same wards, consequently, most cases were related to long-stance carriers. Besides, the continuous entrance of undetected cases from previous admissions, other hospitals, nursing facilities or long-term facilities made the outbreaks difficult to contain, as they could not be rapidly detected. These circumstances made local outbreaks re-emerge systematically in some units.
The active surveillance and screening on admission were implemented since January 2013, and the cohort ward was opened in December 2012. This progressive implementation of control measures recommended by international organisations allowed earlier detection of unrecognised carriers, and consequently, the reinforcement of control measures could be more effective. Furthermore, the generalisation of electronic alerts, as well as the reinforcement on educational measures on hand hygiene and contact precautions, probably fostered the outbreak containment but not achieved the eradication. The CI trend changed from a positive to a negative slope which was maintained for months (Fig. 3). We should also point out that in 2014 Carlos III Hospital joined La Paz Hospital, forming a six-hospital complex. This probably did not affect the number of incident cases since the number of admissions added in 2014 compared with that of 2013 was not significant, and the monthly CI curve continued decreasing with a constant slope. This decrease took several months, which is not surprising, as some reports of CPE outbreaks show that it may take years to achieve some kind of success in tertiary hospitals [Reference Gharbi19–Reference Munoz-Price21].
Detailed analysis of patient registers showed that in 15.6% of previously colonised patients an OXA-48-PE was isolated in a clinical sample later, and some of them developed a clinical infection. Our percentage was similar to the overall risk of infection reported by Tischendorf J et al. among CPE colonised patients (16.5%) [Reference Tischendorf, de Avila and Safdar22], but higher than that reported by Martin et al., who determined that only 5.2% of patients colonised with K. pneumoniae later developed an extra-intestinal infection [Reference Martin23]. The median number of days to detection of clinical samples in previously colonised patients was 20 days, showing that infections emerged more frequently in patients with longer stays and with higher co-morbidity.
Urine was the most frequently isolated clinical sample, which might be explained by the urinary tract infection pathogenesis itself: closeness to the rectal area where colonisation is most frequently detected, and because of constant manipulation for urinary catheterisation.
K. pneumoniae was isolated in 93.6% of the total incident cases (Fig. 2). The high transmissibility of K. pneumoniae, and the ability of blaOXA-48-carrying plasmid for the horizontal gene transfer can facilitate the spread and make outbreaks difficult to contain [Reference Haverkate24].
Another epidemiological feature that is worthy to remark is that 2.3% of the patients had no previous hospital admissions, but had healthcare contact in outpatient clinics or in the Emergency Department. This percentage of CPE colonisation in non-hospitalised patients was similar to the one reported by a study done in Germany. Heudorf et al., in a mandatory reporting system of carbapenem-resistant Gram-negative bacteria, found a 3.9% of patients from out-patient care facilities [Reference Heudorf25].
One of the limitations of our study was the use during the two-year period of cefotaxime-selective-agar to screen OXA-48-PE, as some non-ESBL isolates might be lost. Indeed, now we perform the detection of CPE with a selective medium (chromIDCarba Smart, Biomerieux) to avoid or minimise this problem. Retrospectively, we have measured the incidence of non-ESBL OXA-48-PE in clinical samples in our institution. During the study period, this incidence was nearly 12% therefore, while a proportion of patients colonised with non-ESBL OXA-48-PE might have been lost, we think it would be very low.
Other limitations of this study were adherence to control measures, which were not systematically assessed, and that we only studied cases, so we cannot infer a casual link between results obtained and measures implemented. Nevertheless, this is the first global analysis of incident cases in our hospital complex, and it will be interesting to continue with the study in the future and to assess new interventions and look at longer-term trends. It will provide knowledge to other tertiary hospitals facing this global threat.
Finally, we can conclude that, although we could not eradicate the outbreak, we observed a statistically significant drop on CI after the intervention for OXA-48-PE control, based on patient cohort, active surveillance, electronic alerts and reinforcement of infection control measures in a tertiary hospital.
Acknowledgements
Data presented were obtained as part of the routine work of the Preventive Medicine and Microbiology Departments of Hospital Universitario La Paz.
Ethics approval
The Hospital Ethics Review Committee evaluated and approved the study protocol (PI-2546).
Declaration of interests
The authors declare no conflicts of interest.









