Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in a few unusual pneumonia patients linked to the Wuhan seafood wholesale market in China in December 2019 [Reference Chen1]. However, it soon grew out of China and the Coronavirus disease 2019 (COVID-19) was declared a pandemic on 11 March 2020, and has been reported in 216 countries, areas, or territories [2]. While the epidemic has slowed down in China due to the strict quarantine and preventive regulations, the numbers of COVID-19 patients (i.e. 10021401 as of 28 June, 2020) and confirmed deaths (i.e. 499913 as of 28 June, 2020) are rapidly increasing [2] and have surpassed that of other viruses in the coronavirus family with similar genomes to SARS-CoV-2. For example, SARS which emerged in 2003, infected 8098 patients and caused 774 deaths across 29 countries. The Middle East respiratory syndrome (MERS) which appeared in 2012, led to 2494 patients and 858 deaths across 27 countries [Reference Daga3–6]. The healthcare systems in many countries such as the USA, Spain, Italy, France, UK, Turkey and Iran have been overwhelmed and struggling with the soaring number of patients [7].
Although our understanding of COVID-19's epidemiology is evolving, it is assumed that SARS-CoV-2 is mainly transmitted via droplets and close contacts with people carrying the virus [2]. However, recent reports have also proposed the possibility of the virus being contracted via various surfaces, gastrointestinal transmission [Reference Xiao8] and potentially airborne exposures [2, Reference Lovelace, Higgins-Dunn and Feuer9]. Based on the existing evidence, elderly population, those with suppressed immune systems and underlying metabolic, cardiovascular or respiratory diseases are at an increased risk for adverse outcomes; however, recent reports from outside China, point to a considerable risk of severe outcomes among the general adult population (i.e. <65 years old) [10, Reference Picard11].
As we continue to learn more about COVID-19 and its characteristics, there are many unknowns about its epidemiology such as hospitalisation and recovery-related outcomes that are critical for healthcare system preparedness [Reference AlTakarli12, Reference Goh, Kalimuddin and Chan13]. For example, the mean number of incubation days for COVID-19 varies greatly across the existing literature ranging from 2.5 [Reference Zhang14] to >20 days [Reference Guan15, 16]. Our understanding of time from contracting the disease to recovery or death is even more limited. In this systematic review and meta-analysis, we tried to identify the studies that recruited patients diagnosed with COVID-19 and calculate pooled estimates for several epidemiological and clinical outcomes to help provide an overall picture of the epidemiological characteristics of COVID-19. Findings of this study could help inform the ongoing public health and public policy practices across the world.
Methods
The details of inclusion criteria and our analytical approach were designed a priori and are documented in Open Science Framework (https://osf.io/a3k94/).
Literature search
Following the Systematic Reviews and Meta-Analyses (PRISMA) checklist (see Supplementary Table S1) and the Peer Review of Electronic Search Strategies (PRESS) guideline [Reference Moher17, Reference McGowan18], we searched PubMed, Embase and Google Scholar from 1 December 2019 to 11 March 2020 for studies that measured and reported several characteristics of COVID-19 (e.g. incubation period, hospitalisation, death). Search terms were combined using appropriate Boolean operators and included subject heading terms/keywords relevant to COVID-19 (e.g. novel coronavirus, sars-cov2, coronavirus disease). Please see Supplementary Table S2 for our sample search strategy.
Inclusion criteria
Quantitative studies were included in the review if they reported incubation period of of SARS-CoV-2 as well as time from onset of the symptoms to first medical visit, intensive care unit (ICU) admission, recovery (as defined by studies' authors) or death. Studies were also included if they reported the number of deaths among patients with a confirmed COVID-19 diagnosis. Studies were included in the meta-analysis if they provided data on the above-mentioned outcomes along with their standard error and sample size. Case-reports with a sample size of one were removed from the meta-analysis as they did not provide any dispersion estimate. Studies were not excluded based on language, location or measurement method. Given that this study used secondary data and involved no interaction with humans, no ethics approval was required.
Study selection
Two authors (SJ and NN) completed the abstract and full-text screening, independently. The full-texts of citations that met our inclusion criteria or were unclear were screened by two independent reviewers (SJ and NN). Disagreements over the inclusion of studies were resolved through discussion or by arbitration with the senior author (HS). Duplicate records were excluded.
Data extraction
Data were extracted independently by the two authors (SJ and NN) and discrepancies were resolved through discussion or by arbitration with the senior author (HS). Data were extracted on publication date, study type (e.g. cross-sectional, case-series, cohort), location, sample size, as well as patients' age and sex. We also extracted data on exposure history, X-ray and computed tomography scan findings, symptoms and underlying conditions in addition to the main outcomes of interests including the number of deaths among confirmed COVID-19 patients (i.e. case fatality rate [CFR]), incubation period and time from onset of COVID-19 symptoms to first medical visit, ICU admission, recovery, and death.
Statistical analysis
Meta-analysis was performed using STATA's (V.15.1) metan (for numerical variables) and metaprop (for binary variables) commands. The 95% confidence intervals (CI) for binary variables were computed using the exact binomial method. Heterogeneity between the studies was assessed using both the I2 statistic with a cut-off of 50% and the χ 2 test with P-value <0.10 [Reference Deeks, Higgins, Altman, Higgins and Green19]. As all results turned out to be significantly heterogeneous, we used random-effects models to calculate the pooled point estimate and 95% CI for the CFR, mean time from onset of COVID-19 symptoms to first medical visit, ICU admission, recovery, and death. For the mean incubation period, we estimated 99% CI. We also conducted a random-effects meta-regression using STATA's metareg command to identify the sources of heterogeneity and explored the effect of study-level covariates where data were available (Supplementary Table S3). Meta-regression was considered when there were at least 10 studies included in the meta-analysis [Reference Higgins and Green20]. A two-sided P-value <0.05 was considered as statistically significant.
Results
Participants and study characteristics
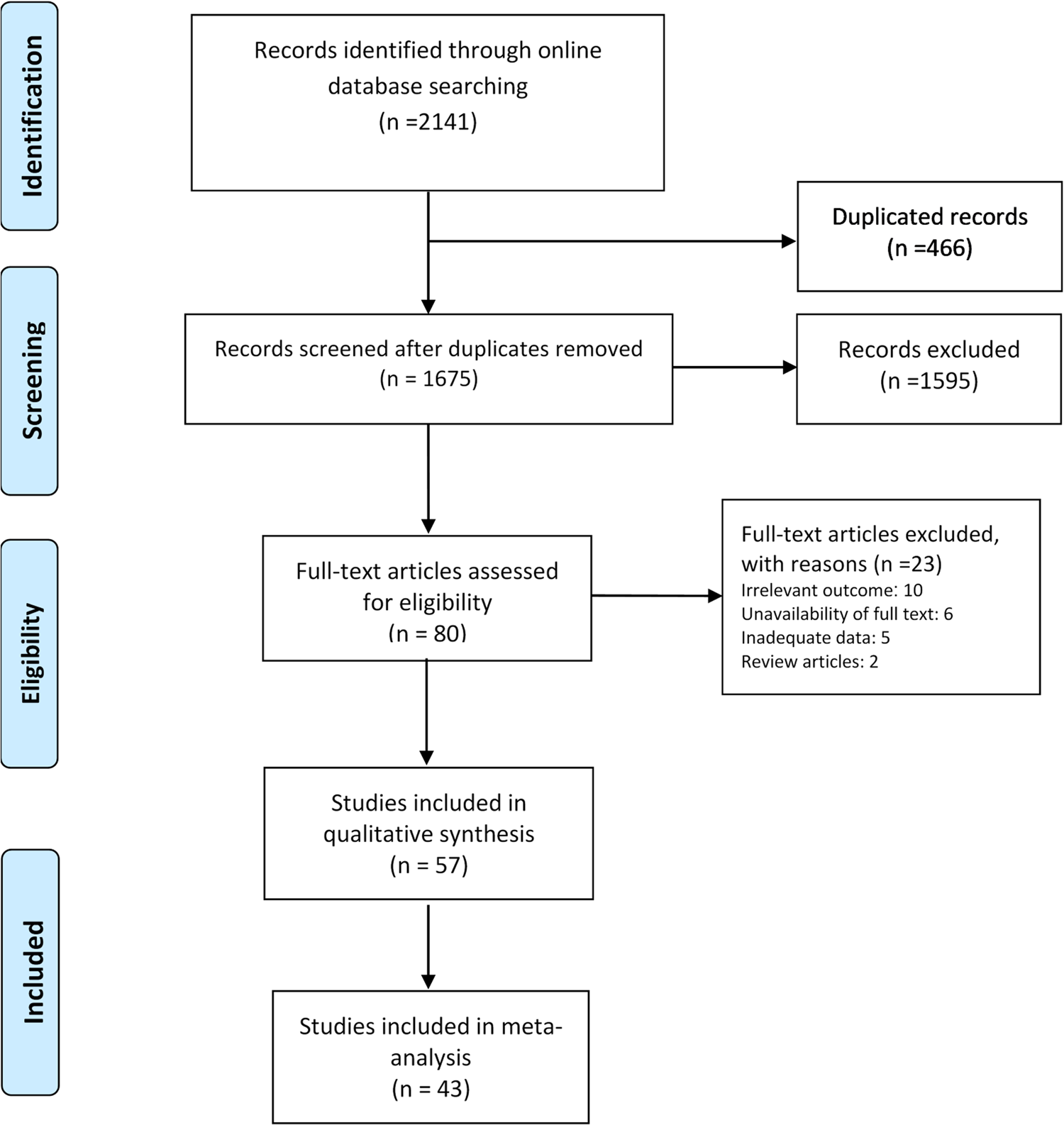
We found a total of 1675 non-duplicate studies, 57 of which were included in the qualitative synthesis and 43 were included in the meta-analysis (Fig. 1). A description of the main characteristics of the included studies is provided in Table 1. The 57 studies included 27 cross-sectional, one case-control, one retrospective cohort and 28 case series/case report studies with sample sizes ranging greatly from one in case-reports to 58 182 for a study in the Hubei province [Reference Dey21]. Inclusion criteria varied greatly across the studies but most study participants were hospitalised patients living or travelling from various provinces in China. Median (range) age of the participants was 46.2 (range: 17 days to 78.5 years) and about 60% were male. Most studies were conducted between January and February 2020. Clinical and epidemiological characteristics of the patients included in the study are presented in Table 2. Among studies that reported exposure history among their participants, most patients were directly or indirectly traced back to the city Wuhan (e.g. lived in Wuhan or had recently travelled to Wuhan) and the Huanan seafood market in Hubei province, China. Several cases of contracting SARS-CoV-2 through close contacts with family members were also reported across the studies. Frequent CT or X-ray findings included thickened texture of the lungs, bilateral focal consolidation, lobar consolidation, ground-glass opacity, patchy consolidation, and unilateral/bilateral pneumonia. Common symptoms reported across the studies included fever, cough, shortness of breath, and fatigue/weakness. Only 15 studies reported some information about the pre-existing conditions of the patients; most of whom had metabolic and cardiovascular underlying conditions.
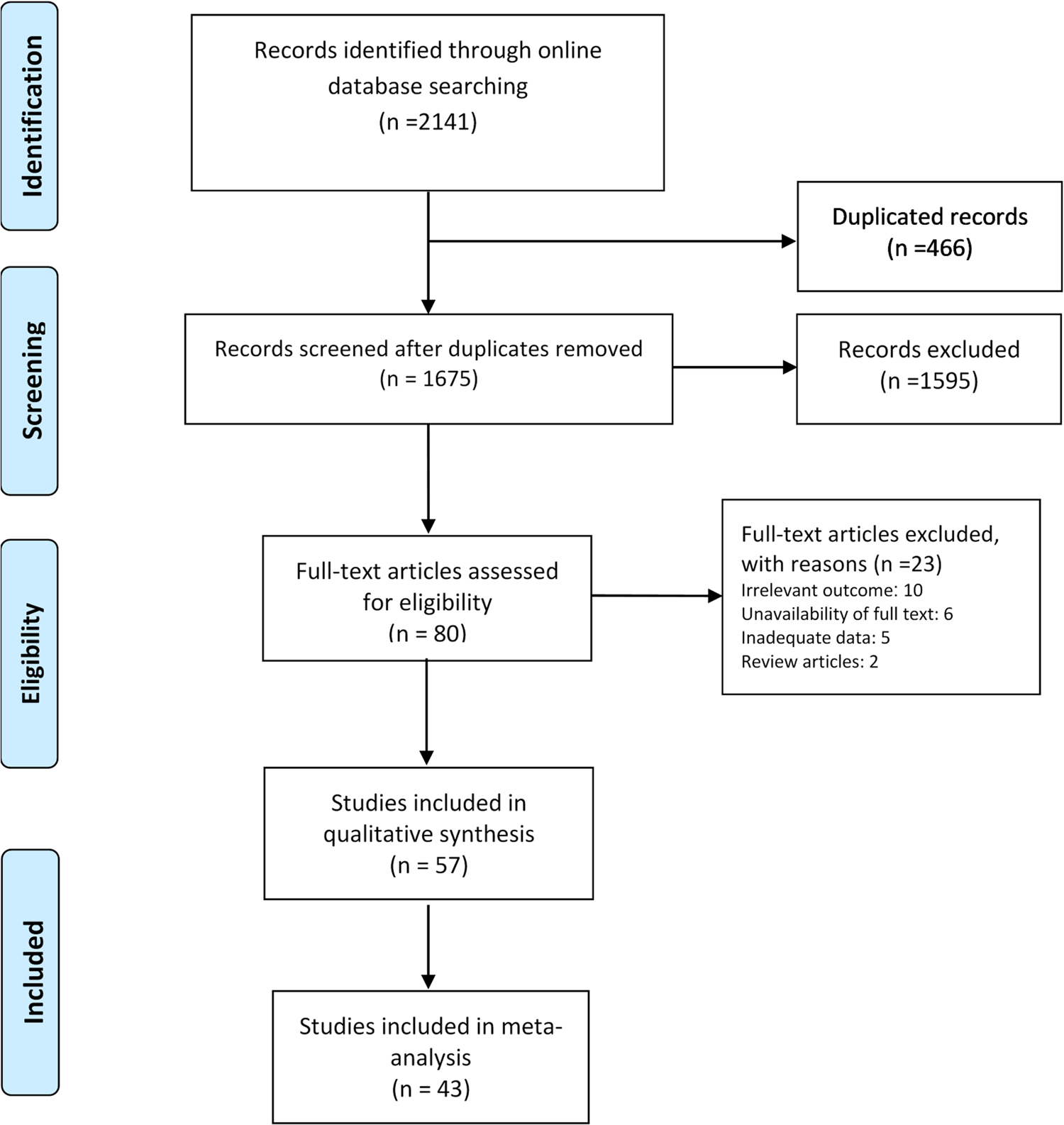
Fig. 1. PRISMA flowchart of screened and included studies.
Table 1. Characteristics of the included studies in the systematic review

a Studies with a sample size less than or equal to four patients were labelled as case-reports [Reference Murad76].
b A 7-year-old-boy and his parents.
c Studies are in press and will be published in future issues of the respective journals.
Table 2. Medical and epidemiological characteristics of the studies included in the systematic review

a Mean (s.e.) day unless specified otherwise.
b Time refers to time from onset of symptoms.
Mean incubation period
The estimated mean incubation period obtained from the included studies and the pooled mean are presented in Figure 2. Out of the 18 studies included in the meta-analysis, 15 were conducted in China. The pooled mean incubation period was 5.68 (99% CI: 4.78, 6.59) days. Heterogeneity testing (I 2 = 98.4%) revealed notable differences among the included studies in the meta-analysis. Multivariate meta-regression results showed no significant differences in incubation period time by country (China vs. others, Adjusted β = 1.76; P-value = 0.375), age (Adjusted β = −1.16; P-value = 0.151) or male percentage of the participants (Adjusted β = −12.35; P-value = 0.058).

Fig. 2. Incubation period of COVID-19.
Mean time from onset of symptoms to first clinical visit
The estimated mean number of days from the onset of COVID-19 symptoms to first clinical visit was 4.92 (95% CI: 3.95, 5.90). As shown in Figure 3, out of the 24 studies included in the meta-analysis, only six were conducted outside China. Heterogeneity testing (I 2 = 98.3%) revealed notable differences among the included studies in the meta-analysis. Multivariate meta-regression results showed no significant differences in time from onset of symptoms to first clinical visit by country (China vs. others, Adjusted β = 1.51; P-value = 0.411), age (Adjusted β = 0.92; P-value = 0.153) or male percentage of the participants (Adjusted β = −2.60; P-value = 0.626).

Fig. 3. Time from onset of symptoms to first clinical visit for COVID-19 patients.
Mean time from onset of symptoms to ICU admission
The estimated mean number of days from the onset of COVID-19 symptoms to ICU admission was 9.84 (95% CI: 8.78, 10.90), an estimate that was derived from one study in Singapore and two studies in Wuhan, China (Fig. 4).

Fig. 4. Time from onset of symptoms to ICU admission for COVID-19 patients.
Mean time from onset of symptoms to recovery
The estimated mean number of days from the onset of symptoms to recovery was reported in seven studies and the resulting pooled mean was 18.55 (95% CI: 13.69, 23.41). Only two studies were conducted in China and the rest were completed in France, South Korea, the UK, Singapore and Japan (Fig. 5).

Fig. 5. Time from onset of symptoms to recovery for COVID-19 patients.
Mean time from onset of symptoms to death
The estimated mean number of days from the onset of symptoms to death was reported in three studies with a pooled mean of 15.93 (95% CI: 13.07, 18.79). All of the studies were conducted in China (Fig. 6).

Fig. 6. Time from onset of symptoms to death for COVID-19.
Case fatality rate
The estimated CFR among COVID-19 patients was reported in 23 studies; most of which included hospitalised patients, three included ICU patients [Reference Huang37, Reference Wang45, Reference Peng60], and none included outpatients. The pooled CFR was estimated as 0.02 (95% CI: 0.02, 0.03) (Fig. 7). Heterogeneity testing (I 2 = 97.6%) revealed notable differences among the included studies in the meta-analysis. Multivariate meta-regression results showed a significant difference in CFR by age (Adjusted β = 0.056; P-value = 0.003).

Fig. 7. Crude fatality rate among COVID-19 patients.
Discussion
We conducted a systematic review and meta-analysis to provide an overview of the epidemiological characteristics of COVID-19 based on the existing evidence as of 11 March 2020. Our findings suggest that COVID-19 has an average incubation period of 5.68 days and there is a lag of 4.92 days from onset of symptoms to the first clinical visit. On average, the symptoms of the patients lasted less than 20 days (18.55 days) before recovery was achieved and the CFR among confirmed COVID-19 patients was 2%, which significantly increased by age. Similar to previous studies [Reference Ji77], fever, dry cough, shortness of breath and fatigue were common symptoms among the patients in the included studies. As expected, history of direct or indirect exposure to Wuhan was frequently reported. The most common radiologic findings were bilateral consolidation and pneumonia [Reference Pormohammad78, Reference Lai79].
We found the average incubation period of COVID-19 infection to be less than 6 days which is broadly consistent with previously reported estimates [Reference Li23, Reference Backer, Klinkenberg and Wallinga29, Reference Liu80, Reference Lauer81]. The right tail of the 99% CI of the incubation period for COVID-19 was less than 7 days (6.59). This finding is of particular interest as there are many uncertainties about the incubation period of COVID-19. For example, both the World Health Organization and Centers for Disease Control and Prevention in the USA suggest an incubation period of 2–14 days. However, single outlier cases as long as 19 [Reference Bai47], 24 [Reference Guan15] or 27 days [16] have been reported; estimates that are possibly reflecting a double exposure. Our findings are of particular importance for quarantine-related policies and planning and suggest that the current 14-day quarantine period might be rather conservative. Indeed, we found that except for one small study from China in Anyang city on a cluster of six patients [Reference Bai47], all other studies reported incubation periods less than 10.6 days; therefore, a shorter period of 14 days would suffice and almost all people exposed to SARS-CoV-2 would show symptoms within 11 days of their initial exposure. All in all, decisions to modify or keep the existing policies need to weigh the costs of extending active quarantine against the potential or costs of missing a few patients with delayed-onset symptoms.
COVID-19 seems to have a longer incubation period than that of other acute respiratory viral infections such as human coronavirus (3.2 days), influenza A (1.43–1.64 days), parainfluenza (2.6 days), respiratory syncytial virus (4.4 days) and rhinovirus (1.4 days) [Reference Lessler82, Reference Nishiura and Inaba83]. Furthermore, the median incubation period for SARS has been estimated as 4.0 days in 2009 [Reference Lessler82], which is considerably lower than what we observed for COVID-19. The longer incubation period of the COVID-19 may be one of the major factors that helps explain its rapid spread in comparison with previous respiratory infection viruses. Other factors contributing to the spread of COVID-19 are the lag between the onset of symptoms and first clinical visit (i.e. 4.92 days) and the high number of asymptomatic COVID-19 patients. These findings suggest that MERS and SARS patients may progress to severe symptoms and respiratory failures [Reference Hui, Memish and Zumla84] much faster than most COVID-19 patients [Reference Lai85].
In comparison to MERS with a fatality rate of 35.67% [Reference Nassar86] and SARS with a fatality rate of 11% [87], we found COVID-19 to have a much lower CFR (2%) that significantly increased by age (5.6% increase for every 10-year increase). Although this estimate is comparable with previous studies [40, 88], it is important to recognise the limitations of calculating fatality rates of COVID-19 while the epidemic is still growing. As most COVID-19 patients remain asymptomatic and may recover without seeking medical care, it is likely that the true CFR among people infected with SARS-CoV-2 could be even lower. On the other hand, the estimated fatality rates reported in most studies need to be interpreted with caution as they are often based on the cumulative number of deaths relative to the number of confirmed cases, while patients who die on a given day have been infected at a much earlier date and this would bias the denominator used to calculate the fatality rate [Reference Baud89].
We acknowledge four main limitations of our systematic review. First, our findings are mainly based on studies that recruited patient from clinics and hospitals and therefore, may be biased towards more severe cases. Moreover, our data might be skewed towards early reporting from provinces in China and outcomes might be different in other countries in the Western context. We are, therefore, aiming to update the review as more data become available in the next 12 months to provide more accurate estimates. Second, many studies did not report the study outcomes by subgroups such as age or sex and we could not report group-specific outcomes. Third, we used the mean and the standard error of the incubation period assuming a normal distribution which may have led to the underestimation of the right tail of the distribution. Lastly, given the urgency of the topic and the heterogeneity of the studies included in the review, we did not conduct the risk of bias or quality assessment of the studies. Given the emerging nature of COVID-19 and the observational study design of most of the available evidence, most studies in the review are at a high risk of bias and the quality of existing evidence is relatively low. Nonetheless, our systematic review of literature provides an insightful picture of the epidemiological characteristics of COVID-19 which could inform ongoing public health and public policy decision makings.
Conclusions
Our findings of the epidemiological characteristics of COVID-19 provide important insight into healthcare systems' prevention and planning efforts. The incubation period (i.e. <11 days in most studies) and the lag between the onset of symptoms and diagnosis (i.e. ~5 days) are longer for COVID-19 compared to other respiratory viral infections including MERS and SARS. Current policies of 14 days of mandatory quarantine for everyone potentially exposed to SARS-CoV-2 might be too conservative and longer quarantine periods might be more justified for extreme cases. As effective vaccination or treatment for COVID-19 are yet to be developed, practising the fundamentals of public health and prevention science such as physical distancing and personal hygiene are critical and need to be emphasised and enforced further to reduce the risk of SARS-CoV-2 transmission.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268820001430.
Acknowledgements
Authors did not receive any fund for this study. MK is a member of Pierre Elliott Trudeau Foundation's COVID-19 impact committee and is supported by the Vanier Canada Graduate Scholarship and the Pierre Elliott Trudeau Foundation Doctoral Scholarship.
Conflict of interest
The authors declare no competing interests.
Data availability statements
All of the data are presented in the paper. The dataset for meta-analysis is available upon reasonable request from the corresponding author (Hamid Sharifi; E-mail: hsharifi@kmu.ac.ir).