INTRODUCTION
Salmonellosis is a bacterial disease often characterized by an acute enterocolitis with sudden onset of nausea, vomiting, abdominal cramps, diarrhoea, headache and fever caused by Salmonella bacteria. Salmonella infection causes more deaths than any other foodborne pathogen in England and Wales [Reference Chin1]. The overall treatment costs are high since more than 5 million cases occur annually in the USA alone, although <1% of salmonellosis cases are reported [Reference Adak, Long and O'Brien2]. In Kazakhastan, similarly to most other countries of the WHO European region, the incidence of salmonellosis is gradually decreasing. There were 6896 registered cases in 1993 and since then the overall incidence of salmonellosis decreased from 42·5/100 000 in 1993 to 13·7/100 000 in 2010 [3].
Associations between various climatic variables and the incidence of salmonellosis [Reference Kovats4–Reference Lake9] or food poisoning [Reference Bentham and Langford10, Reference Bentham and Langford11] have been reported in many countries. The effects of either the temperature alone [Reference Kovats4–Reference Fleury5, Reference Zhang, Bi and Hiller7, Reference Lake9] or in combination with precipitation [Reference Zhang, Bi and Hiller6, Reference D'Souza8] were modelled. In a study using data from five large Australian cities, an increase in temperature of 1°C in the previous month was associated with an increase in the number of cases of salmonellosis ranging between 4·1% in Perth and 11·0% in Brisbane [Reference D'Souza8]. Similar associations, but with a lag of 2 weeks have been reported from Adelaide [Reference Zhang, Bi and Hiller6]. Moreover, while in subtropical Brisbane the temperature lag was 2 weeks, in tropical Townsville the association with temperature was observed in the same month [Reference Zhang, Bi and Hiller7]. In contrast to the Australian studies where a strong positive association was found in all studied settings, in European countries, no obvious pattern was found [Reference Kovats4]. While in Australia, the relationship was approximately linear across the whole temperature range [Reference Zhang, Bi and Hiller6–Reference D'Souza8] with no obvious threshold, certain thresholds above which the effect of temperature is present were found in a few European countries [Reference Kovats4]. In Canada, while positive association was observed between temperature with lags of 0–6 weeks and weekly salmonellosis counts in Alberta, no association was observed in Newfoundland-Labrador [Reference Fleury5]. The threshold values for temperature also varied between countries: while in the Canadian province of Alberta it was −10°C [Reference Fleury5] in European countries it varied between −2°C in the Czech Republic and 13°C in Estonia [Reference Kovats4]. While positive effect between temperature and salmonellosis was observed in several countries, Lake et al. were the first to estimate that in England and Wales the effect of temperature had decreased over time suggesting that adaptation strategies directed at reduction of pathogen concentration in food and improvement of food hygiene could counterbalance the effect of climate change [Reference Lake9].
However, nearly all the studies on the association between climatic variables and salmonellosis have been performed in Australia, North America or the European Union, i.e. in countries with high living standards, almost universal access to clean water, well-developed infection control measures and high food hygiene standards, resulting in low incidence of the disease in these countries. Thus, their results need replication in other settings to allow generalization to less developed areas. A recent study from Russia, where the incidence of salmonellosis is greater and the level of population well-being is lower than in countries where similar studies were performed [Reference Kovats4–Reference Lake9] suggests a positive association between salmonellosis and air temperature in the previous month, although the association with precipitation was less certain [Reference Grjibovski12]. Moreover, all previous studies were performed in settings with sub-Arctic, temperate, subtropical and tropical climates [Reference Kovats4–Reference Grjibovski12] while information from countries with continental, arid or semi-arid climates is still scarce.
This study aims to investigate associations between the number of reported cases of salmonellosis and ambient air temperature in Kazakhstan – a rapidly developing country which covers several climatic zones and has a relatively high incidence of enteric infections.
METHODS
Kazakhstan (Fig. 1) is a former Soviet republic which became an independent state in 1991. Life expectancy in Kazakhstan is among the lowest in the European WHO region with one of the greatest gender gaps in the world: 63·6 years for men and 73·5 years for women in 2009 [Reference Katsaga13]. The study was performed in four administrative units of Kazakhstan (Fig. 1).
Fig. 1. [colour online]. Map of Kazakhstan (source: http://geology.com/world/kazakhstan-satellite-image.shtml).
Astana is the capital of Kazakhstan located in the north-central part of the country in the steppe region on both banks of the Ishim River (51° 10′ N, 71° 26′ E). The population of the city increased from 281 000 in 1999 to 709 000 in 2010 making Astana one of the fastest growing capitals in the world. According to the Köppen-Geiger classification, Astana is located on the border between a humid continental and a semi-arid climate and has cold winters and very warm summers [Reference Peel, Finlayson and McMahon14].
Almaty (formerly Alma-Ata, 43° 16′ N, 76° 53′ E) is the largest city of Kazakhstan (population 1 406 000 in 2000) and was its capital until 1997. The city is located in the transitional zone from the mountain slopes to the plains in the southwest of the country and features a humid continental climate with cold winters and very warm summers [Reference Peel, Finlayson and McMahon14].
North Kazakhstan Province (NKP) is the northernmost administrative unit of the country with a population of 643 700 with 201 500 living in the regional capital of Petropavlovsk (54° 53′ N, 69° 10′ E). NKP has a humid continental climate with very cold and dry winters and warm summers [Reference Peel, Finlayson and McMahon14].
South Kazakhstan Province (SKP) is the southernmost administrative unit of the country with a population of 2 430 000 with 629 600 living in the regional capital of Shymkent (formerly Chimkent, 42° 19′ N, 69° 35′ E). According to the Köppen-Geiger classification SKP features a humid continental climate with warm dry summers and cold winters, although the winter temperatures in SKP are warmer than in other regions of the country [Reference Peel, Finlayson and McMahon14].
Monthly counts of all laboratory-confirmed cases of Salmonella infection in all four regions for the period 2000–2010 were obtained from the National Infectious Diseases Surveillance Centre. Data on a weekly or daily basis were not available and therefore were not used in the study. Data on mean monthly ambient air temperature and accumulated monthly precipitation were collected from the Kazakhstani Hydrometeorological Service (Kazhydromet). For the cities of Astana and Almaty, we used data from the city meteorological stations. For NKP and SKP, the data from stations located in the regional capital cities of Petropavlovsk and Shymkent were used, respectively.
Associations between mean monthly temperature, accumulated monthly precipitation (later called precipitation) and salmonellosis notifications were studied by negative binomial regression to allow for overdispersion in the data [Reference Long and Freese15]. Monthly counts of laboratory-confirmed cases were used as a dependent variable. Given that the effects of high temperature on case counts may be delayed up to 9 weeks [Reference Zhang, Bi and Hiller7], we used mean monthly temperature with lags of 0–2 months. Similarly, the monthly amount of precipitation was included with lags of 0–2 months. Seasonal and year-to-year variations were modelled using indicator dummy variables for each month and year as in previous studies by Kovats et al. [Reference Kovats4]. Robust standard errors were calculated for all estimates to adjust for heterogeneity in the model as described previously [Reference Long and Freese15]. To control for autocorrelation in the outcome variable, first- and second-order autoregressive terms were included in the models where necessary after autocorrelations and partial autocorrelations in the dependent variable were assessed.
In addition, as in several previous studies [Reference Kovats4, Reference Zhang, Bi and Hiller7] a ‘hockey-stick’ model was fitted to the data to ensure comparability of the findings. This model assumes no effect of temperature on salmonellosis counts below the threshold and a linear relationship above the threshold. We used the ‘nl’ program in Stata (StataCorp., USA) to estimate a threshold temperature. This model assesses if there is a change in the slope of the regression line of the relationship between the temperature and the number of cases by iterative procedures across the whole range of temperatures and detects the value of this threshold [Reference Bi, Zhang and Parton16]. Moreover, a curvilinear relationship between temperature, precipitation and the number of notified cases of salmonellosis was modelled by fitting cubic splines with knots spaced at 5°C intervals using the ‘uvrs' estimation program [Reference Royston and Sauerbrei17].
All analyses were performed separately for each setting using Stata v. 10·0 software (Stata Corp.).
RESULTS
Altogether, there were 10 437 laboratory-confirmed cases of salmonellosis during the study period in the four regions. Distribution of cases by region as well as data on monthly temperature and precipitation are presented in Table 1.
Table 1. Minimum, mean and maximum values of monthly temperature and precipitation as well as the number of cases and mean population size (2000–2010) of the four regions of Kazakhstan
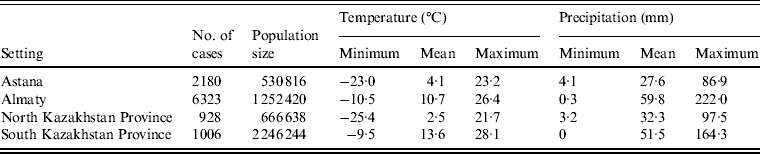
Figure 2 illustrates a clear periodic pattern of salmonellosis notifications in all four locations. The number of cases was decreasing in northern regions, i.e. in Astana and NKP while some increase was observed in southern regions, i.e. in Almaty and SKP. The highest average number of cases occurred in July, the same month as the peak temperature in three of the four areas while in SKP the maximum number of cases was registered in September. The lowest number of cases was observed during the winter months. The seasonal pattern averaged across the 10-year period of observation is presented in Figure 3.

Fig. 2. [colour online]. Number of cases of salmonellosis per month in (a) Astana, (b) Almaty, (c) North Kazakhstan and (d) South Kazakhstan, 2000–2010.

Fig. 3. [colour online]. Seasonal pattern of mean monthly temperature and mean monthly counts of salmonellosis in (a) Astana, (b) Almaty, (c) North Kazakhstan and (d) South Kazakhstan, averaged for each month for the period 2000–2010.
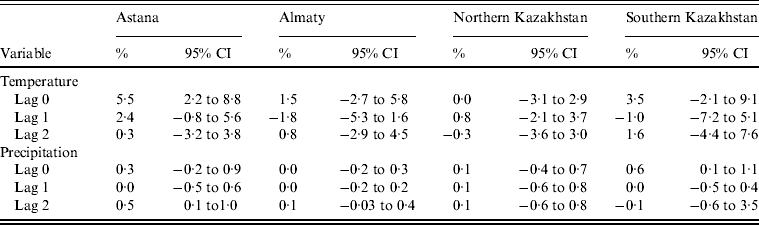
Temperature in the same month was significantly related to the monthly number of salmonellosis cases only in Astana. An increase of 1°C in temperature was associated with an increase in the monthly number of cases by 5·5% (95% CI 2·2–8·8). In addition, in this model an increase in precipitation by 1 mm was associated with a 0·5% (95% CI 0·1–1·0) increase in salmonellosis with a lag of 2 months. There was also an association for precipitation in SKP: a 1 mm increase in precipitation was associated with a 0·6% (95% CI 0·1–1·0) increase in salmonellosis counts in the same months. All associations between temperature, precipitation and the number of salmonellosis cases are presented in Table 2.
Table 2. Percent change in monthly salmonellosis counts per 1°C increase in mean temperature and 1 mm increase in precipitation in four regions of Kazakhstan
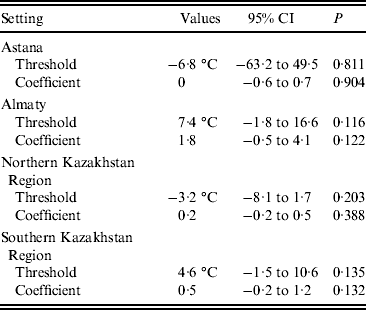
No significant thresholds for the effects of either temperature or precipitation were detected by the hockey-stick models (Table 3). The results of modelling a curvilinear relationship between the studied climatic variables and the monthly number of salmonellosis cases did not reveal a universal pattern. No significant deviations from linearity were detected for any of the climatic variables.
Table 3. Threshold values for mean monthly temperature and coefficients for the association between the temperatures above the threshold (lag 0) and monthly salmonellosis counts in four regions of Kazakhstan, 2000–2010
DISCUSSION
The results of this first study in Kazakhstan and in Central Asia in general suggests a linear relationship between monthly counts of salmonellosis and mean temperature in the same month across the whole temperature spectrum with no threshold values, with the exception of the city of Astana. Moreover, we observed an association between monthly counts of salmonellosis and precipitation with lags of 2 weeks in Astana and 0 weeks in SKP.
In general, our findings are congruent with findings from other countries. Positive associations of similar magnitude between monthly counts of salmonellosis and temperature were observed in five Australian cities in the previous month, while our data suggest an association in the same month. Other Australian studies also reported positive associations between temperature and salmonellosis, although both the effect and lags varied between the settings. In subtropical Brisbane, for example, the effect of temperature was delayed 2 weeks while in tropical Townsville the effect of temperature was observed in the same month as in our study [Reference Zhang, Bi and Hiller7]. Similarly to the Australian studies [Reference Zhang, Bi and Hiller6–Reference D'Souza8], there was no threshold for the effect of temperature. However, no thresholds were found in many European countries – Spain, Switzerland, Slovak Republic, Poland, Scotland and Denmark [Reference Kovats4]. The fact that in three out of four locations the results of our study did not suggest an association between temperature and salmonellosis does not contradict the international literature. For example, no associations between temperature and salmonellosis were observed in Denmark, Slovak Republic and the Canadian province of Newfoundland-Labrador [Reference Kovats4–Reference Fleury5].
As in the study by Kovats et al. [Reference Kovats4], we observed a considerable heterogeneity in the associations between temperature and salmonellosis in different locations. While strong positive association with lag 0 was found in Astana, the associations in Almaty and SKP were less pronounced and did not reach the level of statistical significance due to the relatively low number of cases and short period of observation. At the same time, the data suggest no association between salmonellosis and temperature in NKP. Thus, no specific geographical pattern was observed as in the European study [Reference Kovats4]. Interestingly, the association between the number of salmonellosis cases and temperature in the previous month in Astana is similar to what was observed in another setting in the former Soviet Union – Arkhangelsk, Russia [Reference Grjibovski12]. It was not significant in Astana, however, due to the shorter study period and considerably fewer cases than in the Russian study.
We also found a considerable heterogeneity in the regions in relation to the association between precipitation and salmonellosis in accord with Australian studies. In Adelaide, an inverse association between salmonellosis and rainfall in the same month was observed yet the same authors reported a positive association in Brisbane and Townsville [Reference Zhang, Bi and Hiller6, Reference Zhang, Bi and Hiller7]. In our study, positive association in the same month was found in SKP, while in Astana an association with a lag of 2 months was observed. A positive association in Astana with lag 0, although not reaching the level of significance, was also observed. Contamination of drinking water as a consequence of heavy rainfall is a plausible explanation of the positive association. Growth of Salmonella is greatly reduced in temperatures below 15°C [Reference Doyle and Mazzotta18]. Given that the temperatures in SKP above this threshold hold for 7 months, more than in any other region of Kazakhstan, combined with the fact that SKP is the most rural and poorly developed region with the greatest proportion of migrant workers, transmission through drinking water may seem plausible. An association between salmonellosis and precipitation with a lag of 2 months in Astana, however, requires further investigation.
The main strength of the study is the use of a complete database on all laboratory-confirmed cases of salmonellosis in all four locations from the same reliable source for the whole 10-year period. Another advantage is the use of statistical models applied in other studies to ensure comparability of our results with most of the published studies. Although trigonometric functions have been considered appropriate for controlling for seasonal fluctuations [Reference Zhang, Bi and Hiller6, Reference Zhang, Bi and Hiller7] as well as using polynomials for modelling long-term trends [Reference D'Souza8], we used binary variables for each year and month since they provided better goodness-of-fit for our data. Statistical power was compromised, however, which was reflected in the non-significant results, although absolute values of coefficients for some associations were comparable to larger studies from other settings [Reference Kovats4, Reference Grjibovski12].
The results should be interpreted with caution taking into account potential limitations of the study. Underreporting of the number of cases of salmonellosis is a common limitation of all similar studies using data from passive surveillance systems. Although we used laboratory-confirmed data from a reliable source, it is very likely that the numbers of cases used in this study represent the tip of the iceberg, particularly in SKP. The degree of underreporting varies both between and within countries. For example, only one in 15 cases of salmonellosis is reported in Australia [Reference Hall, D'Souza and Kirk19] and in many industrialized countries only 1% of all cases are registered [Reference Adak, Long and O'Brien2]. Moreover, the registered cases may not be representative of all cases of salmonellosis that occurred during the study period [Reference Tam, Rodrigues and O'Brien20]. The completeness of reporting may vary over time, but using binary dummy variable for each year could at least partly eliminate this effect on the estimates. It is also very unlikely that underreporting is correlated with temperature or precipitation.
Lack of data on outbreaks or incomplete identification of them was considered to be a limitation in several studies [Reference Kovats4, Reference Zhang, Bi and Hiller6–Reference D'Souza8]. We did not have information on the outbreaks of salmonellosis and thus could not exclude cases related to outbreaks. Moreover, the data available for the analysis did not allow identification of travel-related cases. However, in other studies, where this information was available, exclusion of travel-related cases did not influence the results [Reference Kovats4]. Contrary to most other studies in which weekly counts of salmonellosis were used, we used monthly counts because all data for routine reporting from the regional surveillance centres are aggregated by month. However, given the relatively lower number of weekly cases, aggregation seems appropriate and has been used in other studies [Reference Kovats4, Reference Zhang, Bi and Hiller6]. Another limitation of the study is unavailability of data on serotypes of Salmonella which have different sensitivity to climatic variables [Reference Hall, D'Souza and Kirk19]. Although this study does not distinguish between serotypes, it is known that the majority of cases of salmonellosis in Kazakhstan are attributable to S. enteritidis.
Our results may have public health implications in terms of a potential increase in the number of cases of salmonellosis and other enteric infections in the future, particularly if temperatures continue to rise. Temperature may affect contamination at any point along the food chain. Association with temperature with a small or no lag is considered to reflect bacterial growth near the place of consumption while longer lags, e.g. 1 month as in our study, may suggest deficiencies in food hygiene during production, processing and distribution rather than food preparation before consumption [Reference Bentham and Langford10, Reference Bentham and Langford11]. Warmer outdoor temperatures, particularly during the warm season, may also increase the probability of getting salmonellosis and other enteric infections through increased consumption of barbecued food, fresh salads or through outdoor recreational activities, e.g. hiking, swimming or other activities that can increase the likelihood of contacts with Salmonella in the environment. Kazakhstani and Russian traditions of advanced preparation of large amounts of foods combined with the potential for inadequate storage, particularly in rural areas, may also contribute to an increase in the number of cases of salmonellosis. However, the incidence of salmonellosis changes more rapidly than the climate and the baseline incidence of infection is the most important determinant of the effect of climate on enteric infections [Reference Tirado21]. In most countries, including Kazakhstan, a considerable decrease in the incidence of salmonellosis has been observed during the last decades due to the introduction of measures to control S. enteritidis in poultry [Reference Kovats, Menne and Ebi22].
Our results should be replicated in other settings before generalizing the findings to other settings in Central Asia. Moreover, including information on patients' socio-demographic factors, exact dates of symptom onset and Salmonella serotypes are warranted.
CONCLUSIONS
Higher temperatures in the same month are associated with greater monthly counts of salmonellosis in the capital city of Astana, but not in the other studied regions of Kazakhstan. Moreover, a positive association between salmonellosis and precipitation was observed in both Astana and SKP. The results may have implications for future patterns of salmonellosis and other enteric infections in Central Asia with regard to climate change.
ACKNOWLEDGEMENTS
This work has been developed within the WHO/BMU project on protecting health from climate change in Europe, coordinated by Dr Bettina Menne and Dr Joanna Nurse, WHO Regional Office for Europe. We are grateful for the financial support received from Germany.










